Romiplostim Approved to Treat Pediatric Patients with Immune Thrombocytopenia

FDA has approved Amgen’s romiplostim to treat pediatric patients ages 1 year and older with immune thrombocytopenia for a minimum of six months and who have had an insufficient response to corticosteroids, immune globulin or splenectomy.
Human Vaccines Project Launches Study of How the Immune System Responds to the Flu
The Human Vaccine Project aims to understand how the human immune system can develop longer-lasting protection against influenza.
FDA Approves Drug to Treat Acute Myeloid Leukemia

FDA has approved Xospata (gilteritinib) to treat adult patients with relapsed or refractory acute myeloid leukemia with a certain genetic mutation.
Study Says Glaucoma May Be an Autoimmune Disease
A study conducted by MIT and the Massachusetts Eye and Ear has found glaucoma may be an autoimmune disorder.
Tdap Booster Vaccine Receives Expanded FDA Indication
FDA granted Sanofi Pasteur’s Adacel Tdap absorbed vaccine expanded indication to include repeat vaccinations for tetanus, diphtheria and pertussis.
Common Misconceptions Are the Cause for Almost Half of U.S. Adults Skipping the Flu Shot
A new survey shows more than 40 percent of Americans haven’t been vaccinated against the flu and aren’t planning to be due to misconceptions.
Severe Flu Increases Risks for Pregnant Women and Their Babies
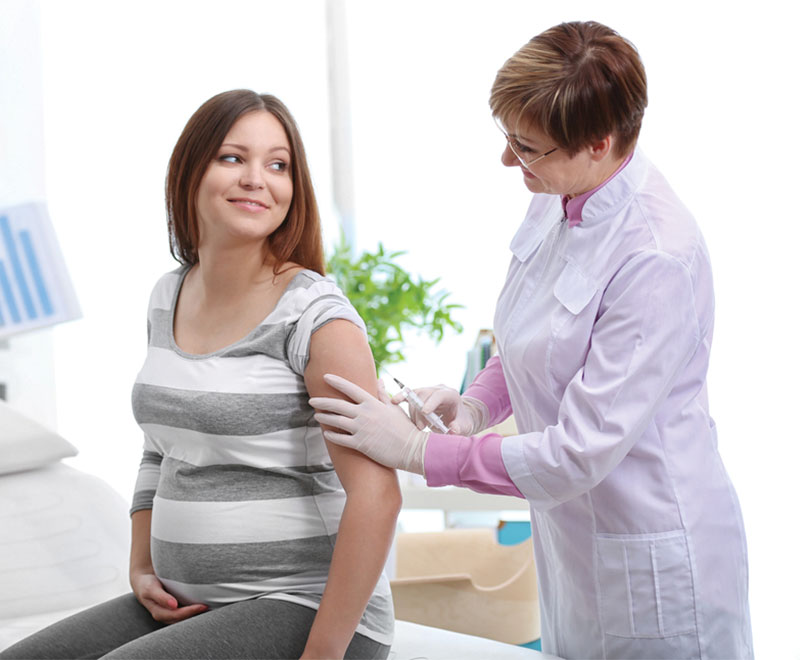
A new study shows pregnant women hospitalized in the ICU with flu are four times more likely to deliver prematurely and four-and-a-half times more likely to have a baby of low birth weight.
$293 Million Awarded by HHS to Expand Primary Healthcare Workforce

The U.S. Department of Health and Human Services’ (HHS) Health Resources and Services Administration (HRSA) has awarded $293 million in awards to primary healthcare clinicians and students through the National Health Service Corps (NHSC) and Nurse Corps programs.
CMS’s New Oncology Care Model Aims to Provide Better Care for Chemotherapy Patients

CMS has developed the Oncology Care Model (OCM) to provide higher quality and more highly coordinated oncology care at the same or lower cost to Medicare.
CMS to Strengthen and Enhance Oversight and Transparency of Medicare Accrediting Organizations

CMS takes steps to enhance and strengthen its oversight and quality transparency of accrediting organizations.