HHS Proposed Significant Cuts to Medicare Payments for 340B Drugs

The U.S. Department of Health and Human Services has proposed 2018 updates to the Medicare hospital outpatient prospective payment system to decrease Medicare Part B payments to hospitals for 340B drugs by almost 30 percent.
Senate Passes CHRONIC Care Act of 2017

The U.S. Senate passed the Creating High-Quality Results and Outcomes Necessary to Improve Chronic(CHRONIC) Care Act of 2017, which aims to improve care for seniors with chronic conditions.
New Drug Approved by FDA to Treat Acute Myelogenous Leukemia
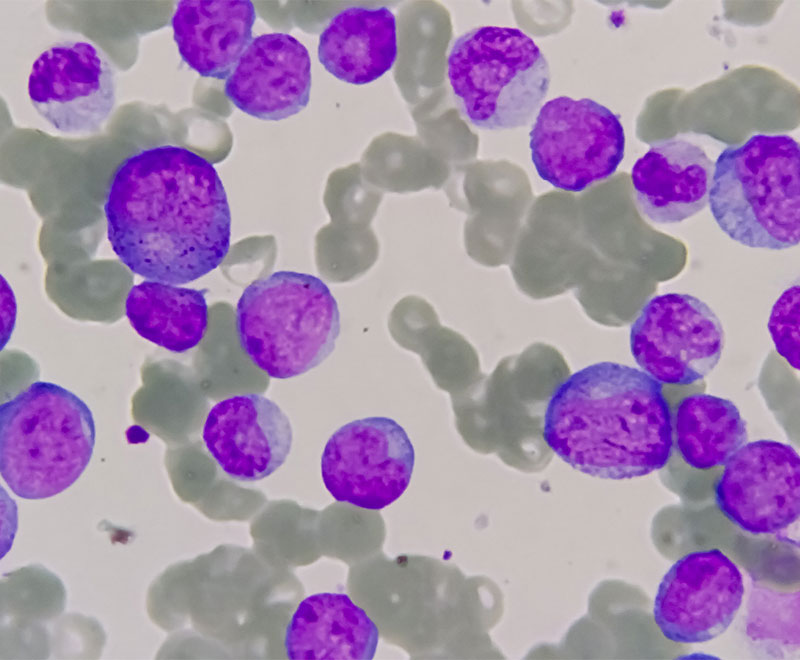
The U.S. Food and Drug Administration has approved Agios Pharmaceuticals’ Idhifa (enasidenib) to treat acute myelogenous leukemia.
FDA Approves First Subcutaneous Therapy to Treat HAE
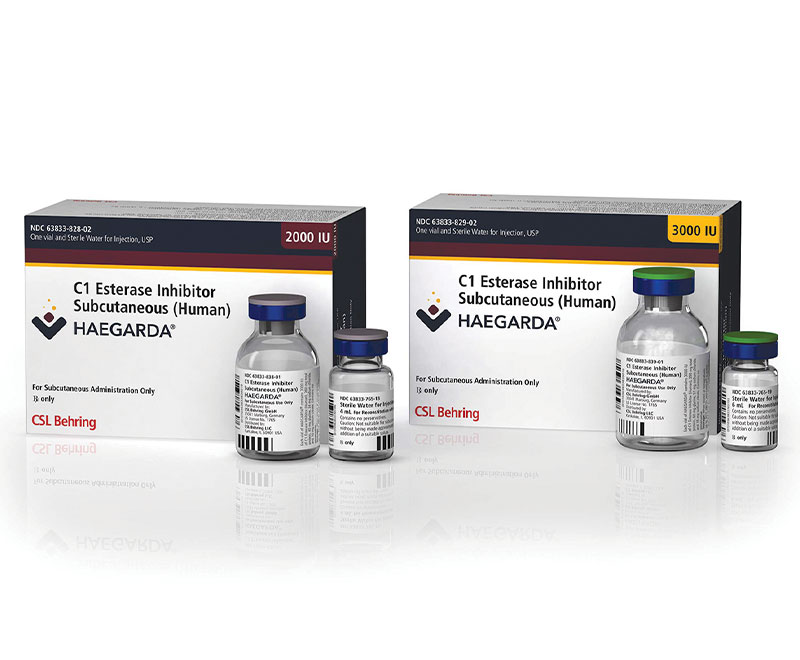
The U.S. Food and Drug Administration has approved CSL Behring’s Haegarda (C1 esterase inhibitor subcutaneous [human]), the first and only subcutaneous therapy indicated for routine prophylaxis to prevent hereditary angioedema attacks in adolescent and adult patients.
CSL Behring ’s Privigen Now Approved to Treat CIDP
The U.S. Food and Drug Administration (FDA) has approved Privigen (immune globulin intravenous [human] 10% liquid) to treat adults with chronic inflammatory demyelinating polyneuropathy (CIDP) to improve neuromuscular disability.
Congress Passes Bills Extending CHIP Funding

The House Energy and Commerce Committee and the Senate Finance Committee passed bills to extend for five years the federal funding forChildren’s Health Insurance Program.
CSL Behring Awards LEAD Grants to Support Patient Rights

CSL Behring has awarded three U.S. bleeding disorder patient organizations Local Empowerment for Advocacy Development (LEAD) grants to help them ensure patients’ voices continue to be heard in their state capitals on legislative and public policy issues.
FDA Approves Mavyret to Treat Adults with Chronic Hepatitis C
The U.S. Food and Drug Administration has approved AbbieVie Inc.’s Mavyret (glecaprevir and pibrentasvir) to treat adults with certain types of hepatitis C.
Clinical Trial of Universal Flu Vaccine Is a Success

Scientists at the National Institutes of Health, Frederick National Laboratory for Cancer Research and the University of Melbourne in Australia tested a new universal flu vaccine that produced good immunity against several different strains of influenza viruses.
New Rules Narrow ACA’ s Contraception Mandate

New rules issued by the U.S. Department of Health and Human Services give employers more leeway to withhold birth control coverage on religious grounds.