Updated C. Diff Guidelines Reflect New Treatment Options and Recommendations
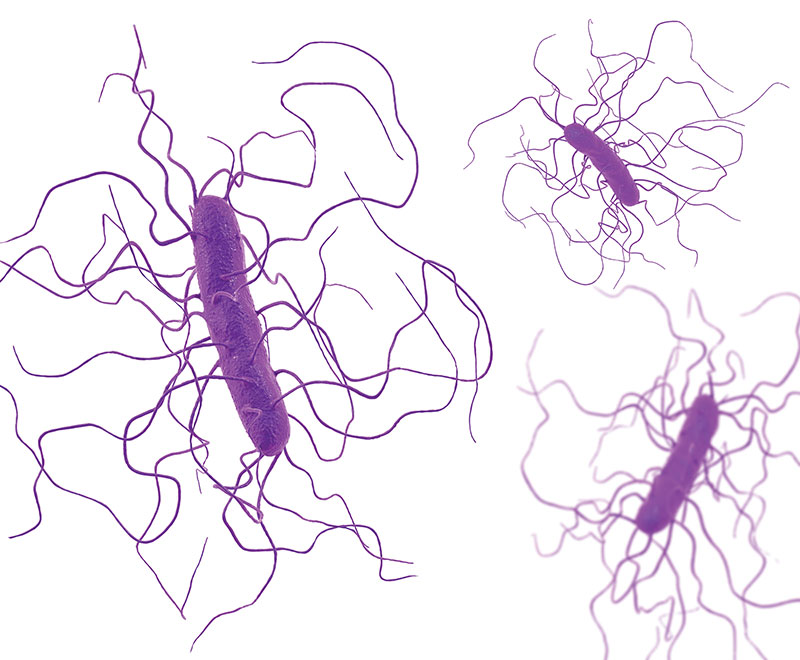
The Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) have updated guidelines for diagnosis and management of Clostridium difficile (C. diff).
Frequency-Escalated Prophylaxis for Severe Hemophilia A Reduces Clotting Factor Usage with Minimal Long-Term Arthropathy
A longitudinal study of Canadian boys with severe hemophilia showed that tailored frequency-escalated prophylaxis results in minimal long-term arthropathy and very good health outcomes, while reducing the quantity of costly clotting factor as compared with standard prophylaxis protocols.
Medical Marijuana and the Opioid Crisis

Can marijuana be used as an adjunct to or substitute for opioids in the treatment of chronic pain
to potentially alleviate the opioid crisis?
The Future of Cord Blood

Once thought to be a useless byproduct, umbilical cord blood now treats more than 80 diseases, and it may soon be used to treat more.
Chronic Human Albumin Therapy Prolongs Survival in Patients with Decompensated Cirrhosis
Results from a clinical trial have led investigators to conclude adding longterm administration of human albumin to conventional treatment inpatients with decompensated cirrhosis appears to prolong survival.
Grifols’ Higher-Potency Rabies Immune Globulin Is Now Available
Grifols’ higher-potency rabies immune globulin (RIG), HyperRAB S/D, was made available to healthcare providers
Updated C. Diff Guidelines Reflect New Treatment Options and Recommendations
The Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) have updated guidelines for diagnosis and management of Clostridium difficile (C. diff).
CSL Behring Will Discontinue Carimune NF in Third Quarter 2018
Discontinuation of the product is due to the preference among healthcare professionals and patients for newer, more advanced immune globulin options.
VONVENDI Approved to Treat Von Willebrand Disease

The U.S. Food and Drug Administration has approved Shire’s VONVENDI, a recombinant von Willebrand factor treatment for perioperative management of bleeding in adults 18 years and older with von Willebrand disease.
Study Finds Antibiotics Can Weaken the Immune System
Researchers have found antibiotics can be counterproductive and weaken the immune system’s ability to fight off bacteria.