Skin Patch Shows Promise for Delivery of Influenza Vaccine
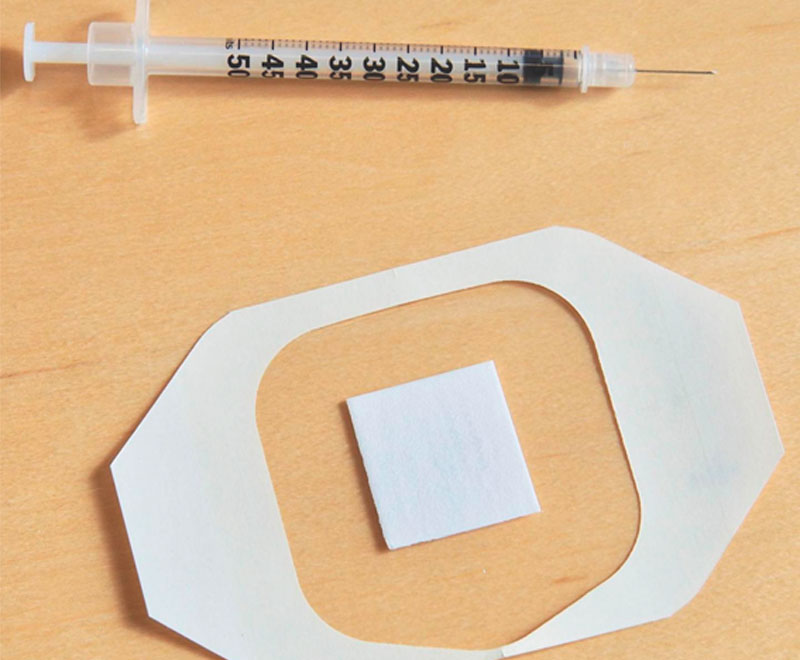
Researchers at the University of Rochester Medical Center in New York City have developed a new type of skin patch that could replace needles as a method to deliver the influenza (flu) vaccine.
NIAID Awards $30 Million to Develop Tuberculosis Vaccine
The awards establish and provide up to seven years of support for three Immune Mechanisms of Protection Against Mycobacterium Tuberculosis (IMPAc-TB) Centers to elucidate the immune responses needed to protect against Mtb infection.
NIH to Evaluate Experimental Adjuvants for Seasonal Influenza Vaccine

The National Institutes of Health (NIH) is conducting an early-stage clinical trial to evaluate the safety and efficacy of two licensed seasonal influenza vaccines administered with or without novel adjuvants.
HPV Vaccine Exceeds Expectations by Providing Herd Immunity
A new study shows the HPV vaccine is far more effective than expected, with benefits extending beyond those who receive the vaccine.
Researchers Develop Vaccine Candidates to Prevent Epstein-Barr Virus
An NIH research team has determined how several antibodies induced by Epstein-Barr virus (EBV) block infection of cells grown in the laboratory.
Stopping Alzheimer’s with a Preventative Vaccine?

Despite the many vaccine candidates undergoing clinical trials, experts doubt an effective vaccine for Alzheimer’s disease is on the horizon.
Sanofi Receives Narrow FDA Approval for Dengue Vaccine
FDA has given Sanofi SA’s dengue vaccine Dengvaxia a narrow approval since the vaccine can cause severe infections in some people.
Campaign Launched to Remind Families Teens Need Second Meningitis Booster Shot

The National Meningitis Association has launched a campaign to encourage more teens to get the meningitis booster.
Connecting with People Who Have Suffered from Diseases Can Change Attitudes Toward Vaccines

A BYU study found introducing vaccine-hesitant people to people affected by vaccine-preventable diseases can decrease vaccine hesitancy.
Genetics Linked to Immunity to Childhood Vaccines

Researchers at the University of Oxford in the United Kingdom have linked several genetic variations with the level of protective antibodies generated following routine childhood immunizations.