Alzheimer’s Research Receives More Funding

The federal government has devoted new funding for Alzheimer’s disease research.
Initiatives to Lower Medicaid Costs and Improve Care

The U.S. Department of Health and Human Services is launching two initiatives to help states save money and better coordinate care for the nine million Americans enrolled in both Medicare and Medicaid.
New Rule Requires Plain Language in Describing Health Plan Benefits and Coverage

The U.S. departments of Health and Human Services, Labor and the Treasury together published new guidelines requiring health insurers and group health plans to provide consumers with plain language information about their health plans.
Partnership for Patients Meeting Participant Goal

Nearly 4,500 organizations — including more than 2,000 hospitals — have pledged their support for Partnership for Patients.
Court Rules for NIH in Stem Cell Research
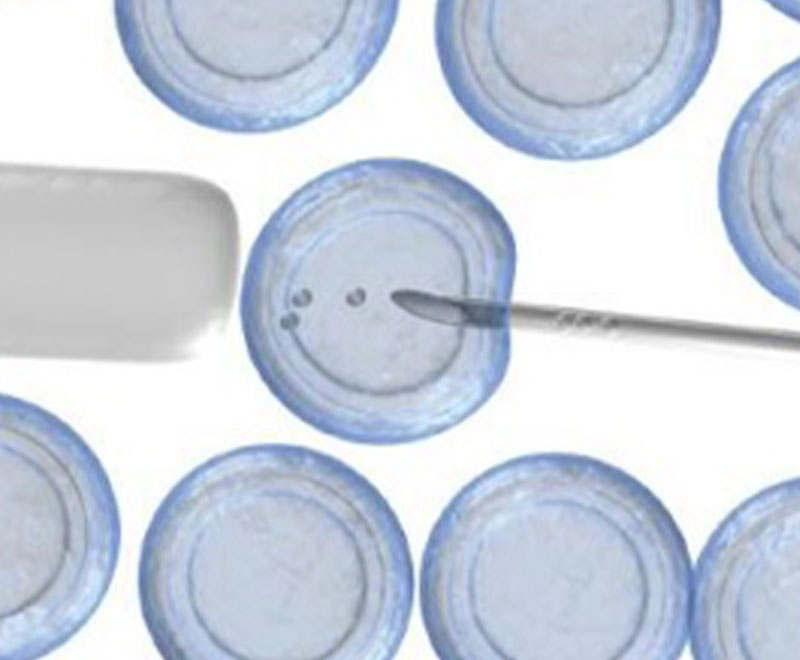
On July 27, 2011, Royce Lamberth, chief judge of the District of Columbia District Court, ruled that the U.S. government can continue funding embryonic stem-cell research.
New Rules Simplify Healthcare Paperwork

The U.S. Department of Health and Human Services has published a new rule for electronic funds transfers in healthcare.
HHS Releases ACO Final Regulations

Responding to concerns about the initial Accountable Care Organization rules, the U.S. Department of Health and Human Services has made several concessions.
Supreme Court to Rule on Healthcare Law Constitutionality

In September, the Justice Department said it would forgo an appeal to the full U.S. 11th Circuit Court of Appeals in Atlanta, which ruled 2-1 in August that the healthcare reform law’s requirement that people buy health insurance is unconstitutional.
CDC to Launch Streamlined Vaccine Tracking System
In April, the Centers for Disease Control and Prevention will launch the final deployment of a system to transform the way the agency distributes vaccines to more than 100,000 U.S.doctors and clinicians.
FDA Approves First MS Oral Treatment

The U.S. Food and Drug Administration has approved Gilenya, the first oral treatment for multiple sclerosis.