Industry News
Research, Science & Manufacturer Updates
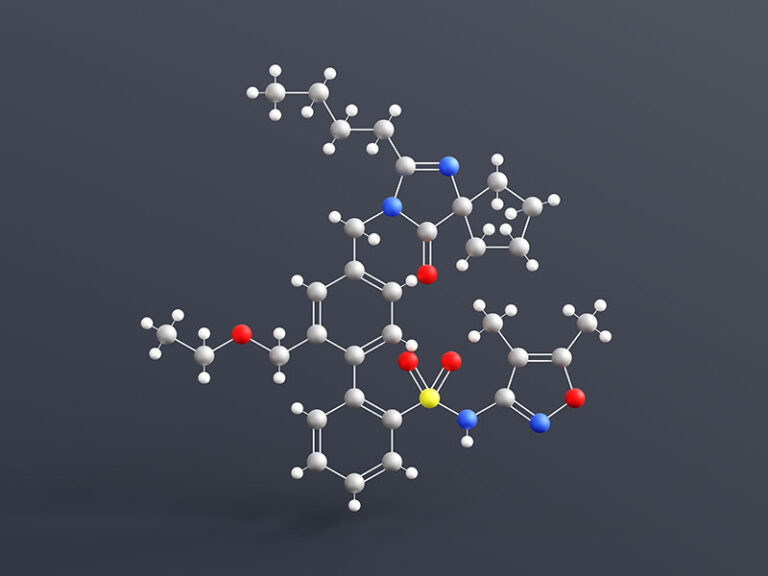
The U.S. Food and Drug Administration has granted accelerated approval for Novartis’ Vanrafia (atrasentan) to reduce proteinuria in adults with primary immunoglobulin A nephropathy (IgAN) at risk of rapid disease progression.
Autism prevalence in the U.S. has increased from one in 36 children to one in 31, according to the Centers for Disease Control and Prevention’s (CDC) latest Autism and Developmental Disabilities Monitoring (ADDM) Network survey.
A recent study found there is significantly higher intravenous immune globulin (IVIG) resistance among children who contracted COVID-19 before developing Kawasaki disease
The University of Toledo will receive $473,632 from the National Institute of Dental and Craniofacial Research to explore how the body’s immune system responds to the condition known as oral thrush.
Researchers at Moffitt Cancer Center have found that tapping into the body’s own immune system and activating a type of immune cell known as B cells could be the key to boosting the effectiveness of tumor-infiltrating lymphocyte, or TIL therapy.
A draft budget proposal circulating among federal officials suggests a cut in the U.S. Department of Health and Human Services’ discretionary spending by as much as one-third, or tens of billions of dollars.
The U.S. Department of Health and Human Services (HHS) and the National Institutes of Health (NIH) have announced the development of the next-generation, universal vaccine platform, Generation Gold Standard, using a beta-propiolactone (BPL)-inactivated, whole-virus platform.
Moderna’s experimental mRNA-based influenze (flu) vaccine produced a stronger immune response than a currently available vaccine in a late-stage trial, clearing a path forward for the product and the company’s separate combination flu and COVID vaccine.
The U.S. Food and Drug Administration (FDA) has approved GAMMAGARD LIQUID ERC [immune globulin infusion (human)] with less than or equal to 2 µg/mL IgA in a 10% solution, the only ready-to-use liquid immunoglobulin (IG) therapy with low immunoglobulin A (IgA) content, as replacement therapy for individuals 2 years of age and older with primary immunodeficiency (PI).
A group of outside advisers to the Centers for Disease Control and Prevention (CDC) voted 5-2 to recommend the use of Merck’s new antibody vaccine, Enflonsia (clesrovimab), that can protect babies from respiratory syncytial virus (RSV).
A new clinical trial shows a single shot of a long-lasting influenza (flu) drug may protect people for an entire season, and it might do so more effectively than vaccines.
Grifols announced positive results from its Phase II/III clinical trial evaluating the efficacy and safety of Flebogamma 5% DIF (intravenous immune globulin [IVIG]) to treat patients with post-polio syndrome, which demonstrated a significant improvement in distance walked compared to placebo.