Industry News
Research, Science & Manufacturer Updates
Washington Report Articles
The Centers for Medicare and Medicaid Services began charging a new application fee to all institutional providers.
As the struggle over the details of the Affordable Care Act persists, changes continue to be made.
A new law is being proposed that would allow Medicare patients who have a chronic condition to review all their medications in one-on-one sessions with pharmacists, helping them to stick to their drug regimen.
Twenty-one Republican governors signed a letter sent in February to U.S.Department of Health and Human Services Secretary Kathleen Sebelius asking for more flexibility for states as they put together their own health exchanges.
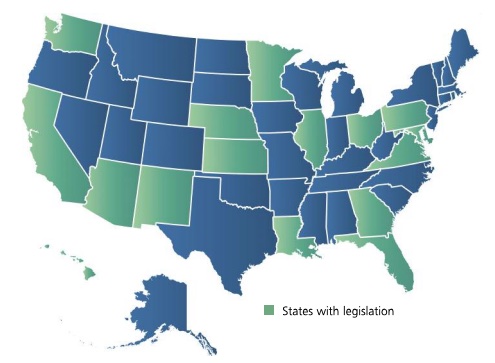
The introduction of legislation to ensure access by patients to high-cost therapies continues to increase throughout the country.
The U.S. Department of Health and Human Services has announced it will provide $750 million to fund new prevention and public health programs, made available through the new healthcare law’s Prevention and Public Health Fund.
As of April 2011, many cuts to healthcare spending are being proposed and expected.
The House Judiciary Committee is working to pass medical tort reform legislation.
In August 2010, the Centers for Medicare and Medicaid Services proposed to change the reimbursement rate of separately payable, non-pass-through drugs and biologicals.
In Massachusetts, a panel of state officials and healthcare executives moved toward global payments by drafting a first-in-the-nation blueprint for scrapping current fee-forservice payments.
Specialty medications costing more than $600 per year are now classified under two new tiers of Medicare Part D drug benefit plan.
Many questions remain unanswered about the healthcare reform recently enacted into law, but here's what we do know.