Revita System Granted Breakthrough Device Designation by FDA

The U.S. Food and Drug Administration has granted breakthrough device designation to Revita System (Fractyl Health Inc.) for the maintenance of weight loss after discontinuation of glucagon-like peptide-1 medications.
FDA Approves Blood Test to Detect Colon Cancer
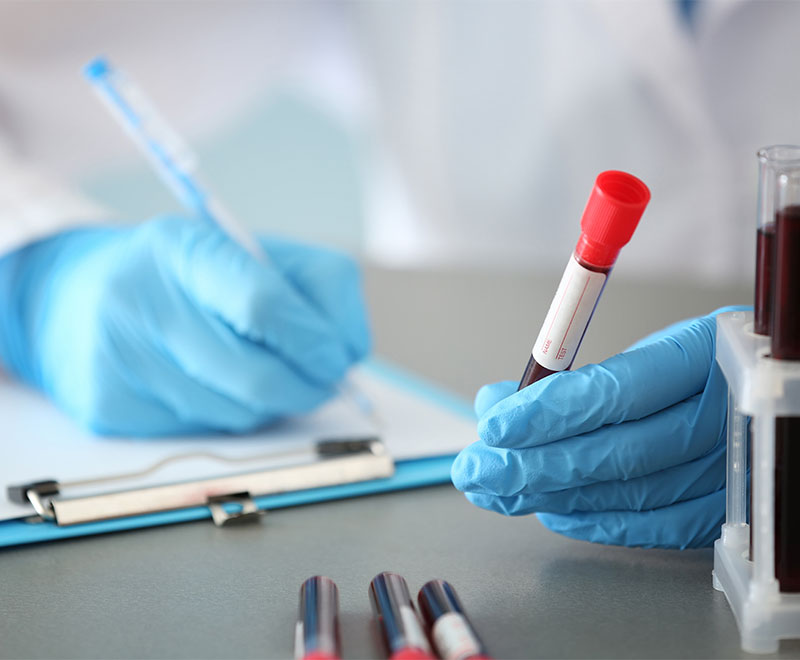
The U.S. Food and Drug Administration has approved a blood test to screen for colorectal cancer in individuals aged 45 and older with an “average risk” of colon cancer.
Deuruxolitinib Approved by FDA to Treat Severe Alopecia
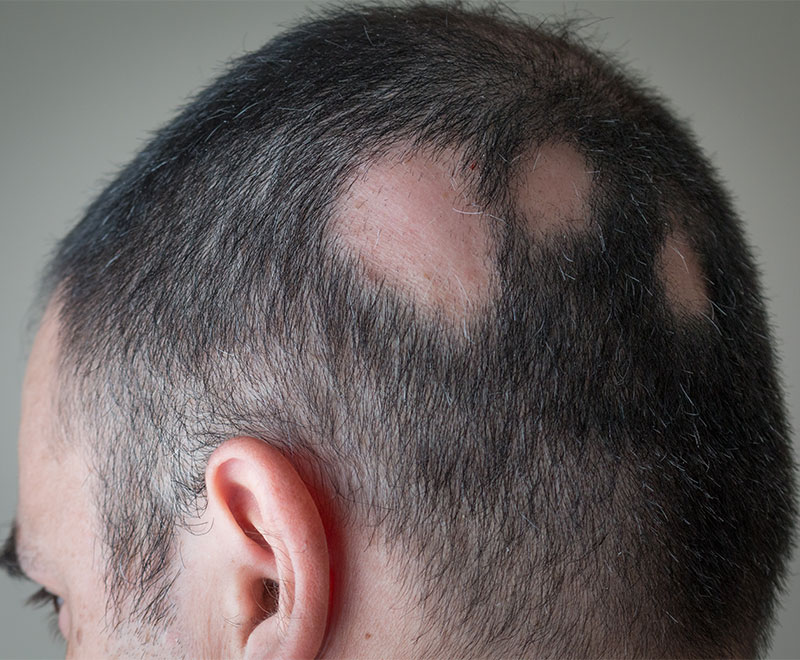
Sun Pharma’s LEQSELVI (deuruxolitinib) has been approved by the U.S. Food and Drug Administration to treat severe alopecia areata in adults.
FDA Approves Epysqli, Second Biosimilar to Soliris

Samsung Bioepis’ biologics license application for Epysqli (eculizumab-aagh) as a biosimilar to Soliris (eculizumab) has been approved by the U.S. Food and Drug Administration (FDA) to treat patients with paroxysmal nocturnal hemoglobinuria (PNH) to reduce hemolysis and atypical hemolytic uremic syndrome (aHUS) to inhibit complement-mediated thrombotic microangiopathy.
Drug to Treat Acute Pancreatitis Granted Fast Track Designation by FDA
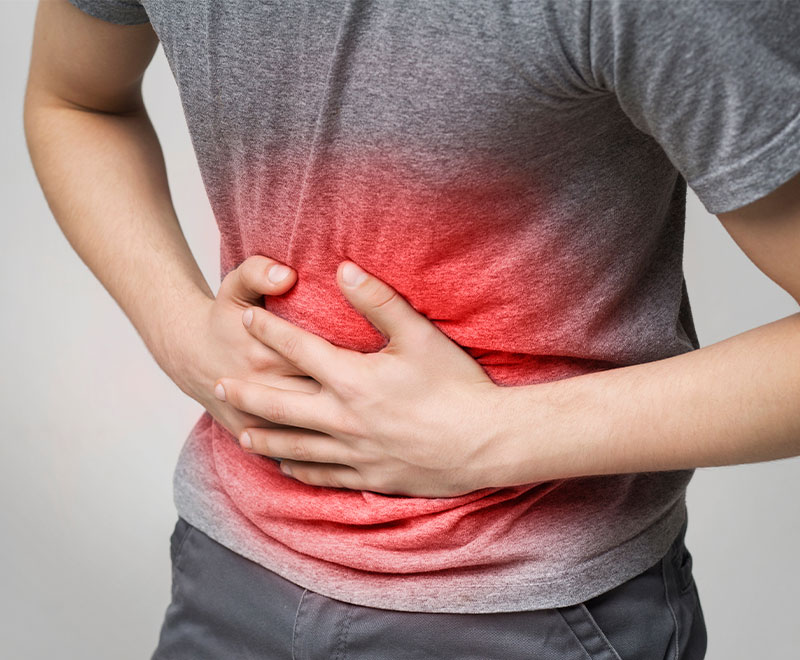
Panafina Inc.’s investigational new drug, RABI-767, has been granted fast track designation by the U.S. Food and Drug Administration (FDA) to treat patients with acute pancreatitis predicted to progress to severe disease.
FDA Approves Yimmugo, an IVIG Product, to Treat Primary Immunodeficiencies
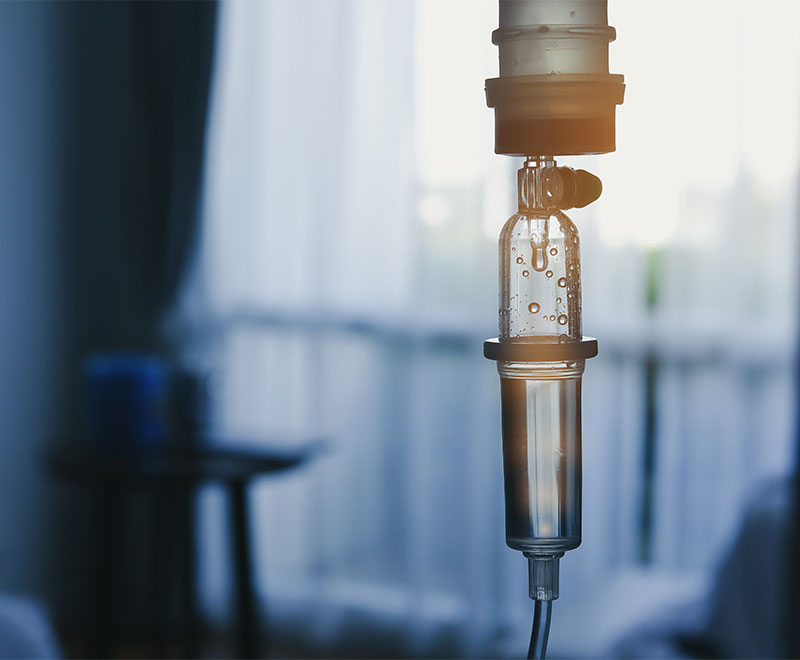
Biotest has received approval from the U.S. Food and Drug Administration for Yimmug to treat primary immunodeficiencies in patients 2 years and older.
First Treatment Approved for Genetic Clotting Disorder
The U.S. Food and Drug Administration has approved a recombinant ADAMTS13 protein product (Adzynma) as a prophylactic or on-demand enzyme replacement therapy for patients with congenital thrombotic thrombocytopenic purpura.
FDA Approves Zepbound as a New Weight-Loss Drug

The U.S. Food and Drug Administration has approved Eli Lilly’s tirzepatide medication, which is branded as Mounjaro for diabetes, under a new brand for weight loss as well.
Complement C5 Inhibitor Zilucoplan Is Approved by FDA to Treat MG

UCB Pharma’s investigational agent zilucoplan, a complement C5 inhibitor, to treat patients with myasthenia gravis has been approved by the U.S. Food and Drug Administration under the market name Zilbrysq.
FDA Approves Biosimilar to ACTMRA

The U.S. Food and Drug Administration has approved Biogen’s tocilizumab-bavi (TOFIDENCE), a biosimilar option referencing ACTMRA, to treat moderately to severely active rheumatoid arthritis (RA), polyarticular juvenile idiopathic arthritis (PJIA) and systemic juvenile idiopathic arthritis (JIA).