Large-Dose Albumin Supplementation Does Not Reduce Mortality or Need for Critical Care Interventions: Retrospective Review
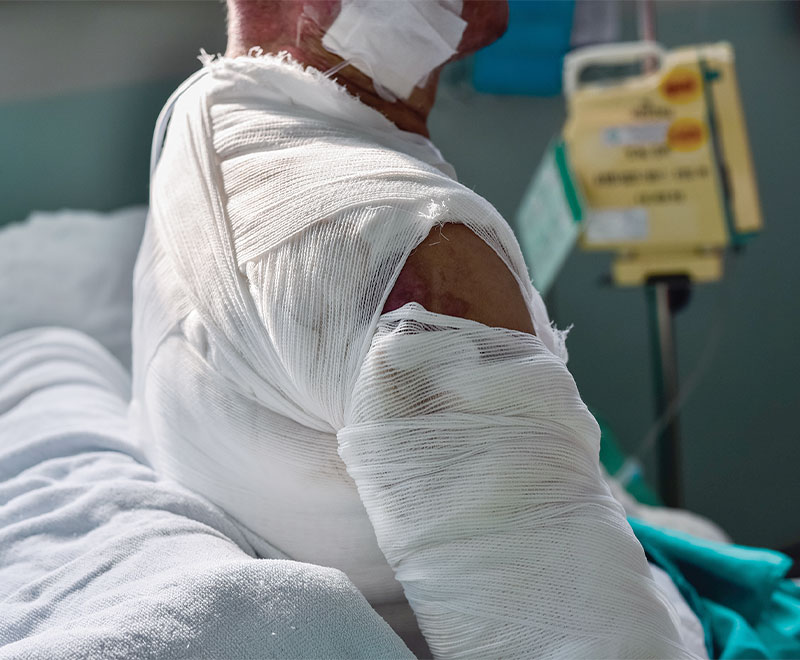
Investigators conducted a retrospective review of 38 cases treated at Taipei Veterans General Hospital between January 2007 and December 2018 to determine whether more aggressive albumin supplementation can benefit major burn patients with persistent hypoalbuminemia.
Bleed Protection with up to 14-Day Dosing of Long-Acting Recombinant Factor IX Product in Selected Children with Hemophilia B
In pharmacokinetic studies, a recombinant fusion protein genetically linking human coagulation factor IX with human albumin (rIX-FP) (IDELVION, CSL Behring) has been shown to have an approximately five-fold longer half-life compared with standard recombinant factor IX products.
Measles Virus Destroys Immune System’s Memory of Past Infections
Two studies were conducted to determine whether measles infection causes long-term damage to immune memory.
Study of Kevzara from Severe to Critical COVID-19 Patients

Following a review by the Independent Data Monitoring Committee (IDMC) of preliminary results from the Phase II portion of an ongoing Phase II/III trial evaluating Kevzara (sarilumab), the trial was amended so only critical patients continue to be enrolled to receive Kevzara 400 mg or placebo.
Novartis Initiates Phase III Trial of Ilaris to Treat COVID-19 Patients with Pneumonia
Novartis has initiated a Phase III clinical trial to examine the efficacy of utilizing canakinumab (Ilaris), an interleukin (IL)-1β blocker, to treat a type of severe immune overreaction called cytokine release syndrome (CRS) in people with COVID-19 pneumonia.
Sandoz Launches Hyrimoz, Biosimilar to Humira

Sandoz has announced that the citrate-free high-concentration formulation of its biosimilar Hyrimoz (adalimumab-adaz) injection became available in the United States starting July 1, 2023.
FcRn Antagonist Boosts Platelet Counts, Reduces Bleeding Risk in Patients with Immune Thrombocytopenic Purpura

Efgartigimod, an investigational human IgG1 Fc fragment, induced a rapid reduction in total IgG levels and clinically relevant increases in platelet counts in patients with primary immune thrombocytopenia (ITP), according to findings from a Phase II, placebo-controlled multinational study.
High-Titer Inhibitors Reduced in PUPs with Severe Hemophilia A Treated with Fourth Generation Recombinant Factor VIII

A study in previously untreated patients (PUPs) with severe hemophilia A found replacement therapy with a fourth-generation recombinant factor VIII (FVIII) product was associated with an incidence of high-titer inhibitors similar to the rate seen in a landmark trial evaluating plasma-derived FVIII products.
Study Finds Safe Treatment for Children with Severe Hemophilia A with Inhibitors

Researchers have found combining immune tolerance induction (ITI) with Hemlibra (emicizumab, Roche) is a feasible and safe way of treating children with severe hemophilia A.
FDA Expands Indication for Octapharma’s WILATE to Hemophilia A

The U.S. Food and Drug Administration (FDA) has approved WILATE for treatment of adults and adolescents with hemophilia A for routine prophylaxis.