Study Shows Antabuse Lowers Risk of Death from Cancer

A nationwide epidemiological study showed cancer patients who continuously used disulfiram (Antabuse), a drug prescribed to alcoholics to prevent them from drinking, have a lower risk of death from cancer compared to those who stopped using the drug once diagnosed.
Are Drugs Really Overpriced?

Pricing of drugs is far more complex than the big, bad pharma price-gouging narrative.
New Drug Approved by FDA to Treat Acute Myelogenous Leukemia
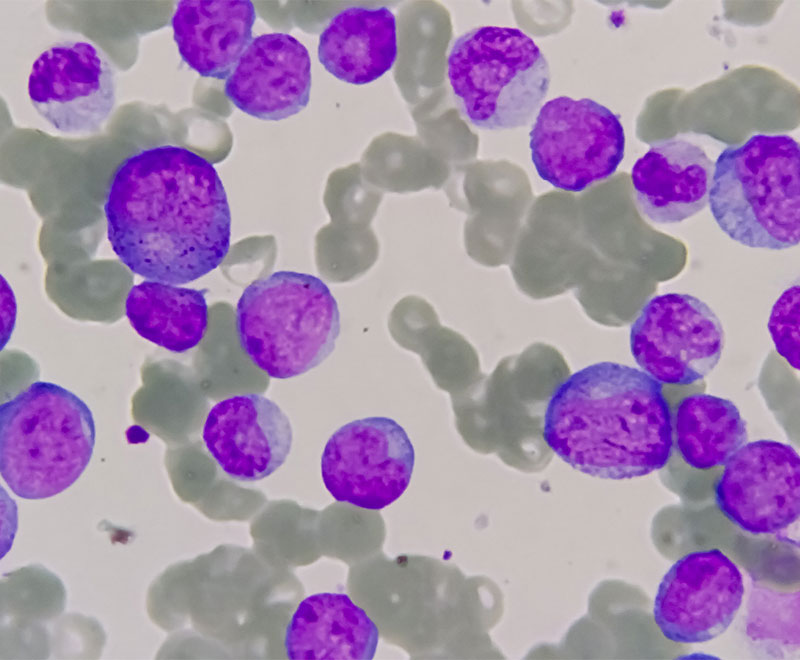
The U.S. Food and Drug Administration has approved Agios Pharmaceuticals’ Idhifa (enasidenib) to treat acute myelogenous leukemia.
FDA Approves First Subcutaneous Therapy to Treat HAE
The U.S. Food and Drug Administration has approved CSL Behring’s Haegarda (C1 esterase inhibitor subcutaneous [human]), the first and only subcutaneous therapy indicated for routine prophylaxis to prevent hereditary angioedema attacks in adolescent and adult patients.
CSL Behring ’s Privigen Now Approved to Treat CIDP
The U.S. Food and Drug Administration (FDA) has approved Privigen (immune globulin intravenous [human] 10% liquid) to treat adults with chronic inflammatory demyelinating polyneuropathy (CIDP) to improve neuromuscular disability.
FDA Approves Mavyret to Treat Adults with Chronic Hepatitis C
The U.S. Food and Drug Administration has approved AbbieVie Inc.’s Mavyret (glecaprevir and pibrentasvir) to treat adults with certain types of hepatitis C.
New Rules Narrow ACA’ s Contraception Mandate

New rules issued by the U.S. Department of Health and Human Services give employers more leeway to withhold birth control coverage on religious grounds.
Expanding Uses of IVIG
Researchers are taking a closer look at intravenous immune globulin for its potential to stop the progression of multiple complex conditions from lupus to multiple sclerosis.
Kedrion Biopharma and Kamada Receive FDA Approval for KEDRAB
The U.S. Food and Drug Administration has approved Kedrion Biopharma’s and Kamada’s KEDRAB (rabies immune globulin [human]) for passive, transient postexposure prophylaxis of rabies infection.
Cutting-Edge Pediatric Cancer Therapy Approved by FDA

Novartis’ Kymriah (tisagenlecleucel) has been approved by the U.S. Food and Drug Administration to treat pediatric acute lymphoblastic leukemia.