FDA Approves Sandoz’s Ziextenzo as 24th Biosimilar

The U.S. Food and Drug Administration (FDA) has approved Ziextenzo (pegfilgrastim-bmez), the 24th biosimilar approval in the U.S.
CMS Launches Healthy Adult Opportunity Demonstration Initiative

The Centers for Medicare and Medicaid Services (CMS) has launched the Healthy Adult Opportunity (HAO) optional demonstration initiative to give states tools to design innovative health coverage programs for adult beneficiaries, while holding states accountable for results and maintaining strong protections for the most at-risk populations.
Medicare Will Now Cover Acupuncture for Chronic Low Back Pain
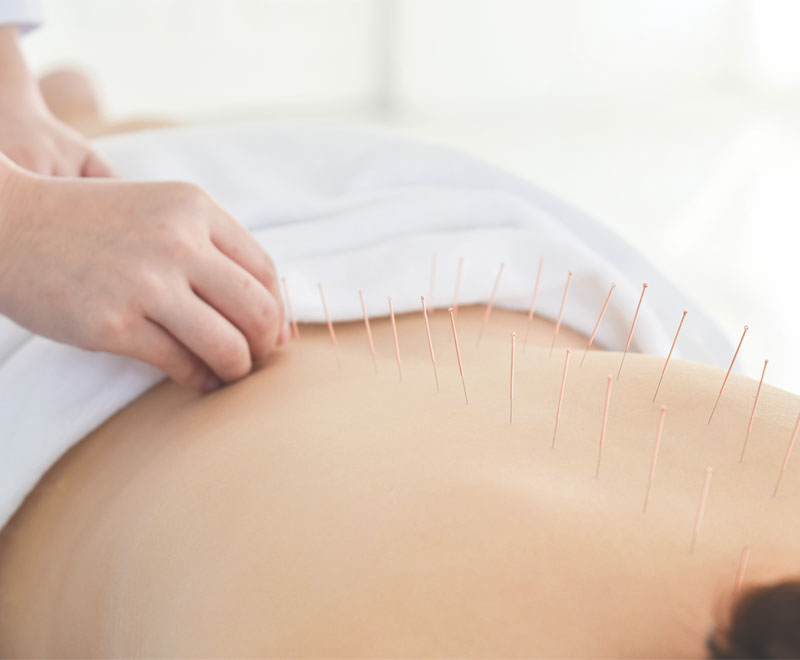
The Centers for Medicare and Medicaid Services (CMS) finalized a decision to cover acupuncture for Medicare patients with chronic low back pain.
Finalized Health Care Price Transparency Rule Unveiled

New rule requires hospitals to share previously obscured price information, including discounts for cash-paying patients and rates negotiated with insurers.
FDA Initiates Coronavirus Medical Countermeasures
The U.S. Food and Drug Administration (FDA) is taking critical actions to advance development of novel coronavirus medical countermeasures.
NIAID Awards $30 Million to Develop Tuberculosis Vaccine
The awards establish and provide up to seven years of support for three Immune Mechanisms of Protection Against Mycobacterium Tuberculosis (IMPAc-TB) Centers to elucidate the immune responses needed to protect against Mtb infection.
NIH to Evaluate Experimental Adjuvants for Seasonal Influenza Vaccine

The National Institutes of Health (NIH) is conducting an early-stage clinical trial to evaluate the safety and efficacy of two licensed seasonal influenza vaccines administered with or without novel adjuvants.
New Rabies Calculator Helps Providers Treat Patients Exposed to Rabies

Kedrion Biopharma has created the free, easy-to-use KEDRAB Dose Calculator to help healthcare providers treat rabies exposures.
Octapharma Introduces New SCIG Product and IgCares Program

Octapharma introduced its newest product, Cutaquig (immune globulin subcutaneous [human] 16.5% solution) at the Immune Deficiency Foundation (IDF) National Conference in June.
FDA Approves Octaplas to Treat Pediatric Patients Who Require Multiple Coagulation Factor Replacement
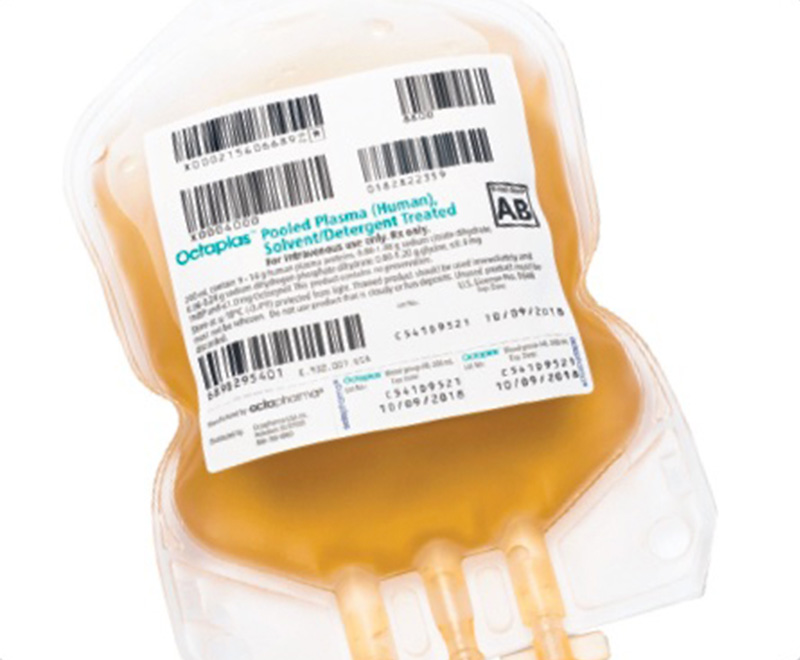
A revised product label for Octapharma USA’s Octaplas (pooled plasma [human] solvent/detergent treated solution for intravenous infusion) to treat critically ill pediatric patients who require replacement of multiple coagulation factors has been approved by the U.S. Food and Drug Administration (FDA).