New Type of Blood Test Can Identify Kids at Risk of Diabetes
Scientists at Kings College London have discovered a novel link between lipids and disorders affecting children’s metabolism that may provide an early warning system for obesity-related problems such as type 2 diabetes and liver and heart disease. The researchers say this could assist medical professionals in identifying early disease indicators in children more quickly and […]
New Drug Approved for Children with Severe Immune Disease

Remestemcel-L, a new treatment for children ages 2 months and older with steroid-refractory acute graft-versus-host disease, has been approved by the U.S. Food and Drug Administration.
New Blood Test for Cancer Patients Makes Treatment Safer and More Effective
Scientists from RMIT University and the Doherty Institute in Australia have developed a new blood test that could screen cancer patients to help make their treatment safer and more effective.
Nasal Swab May Predict Severity of COVID-19

New research suggests autoantibodies in the nasal cavity may predict the severity of COVID-19 disease.
mRNA Cancer Therapy Boosts Immune Response

An investigational, individualized neoantigen therapy, with personalized encoded mRNA, has demonstrated potential to enable patients’ immune systems to target cells that cause cancer.
Nasal COVID-19 Vaccine Halts Transmission of SARS-CoV-2
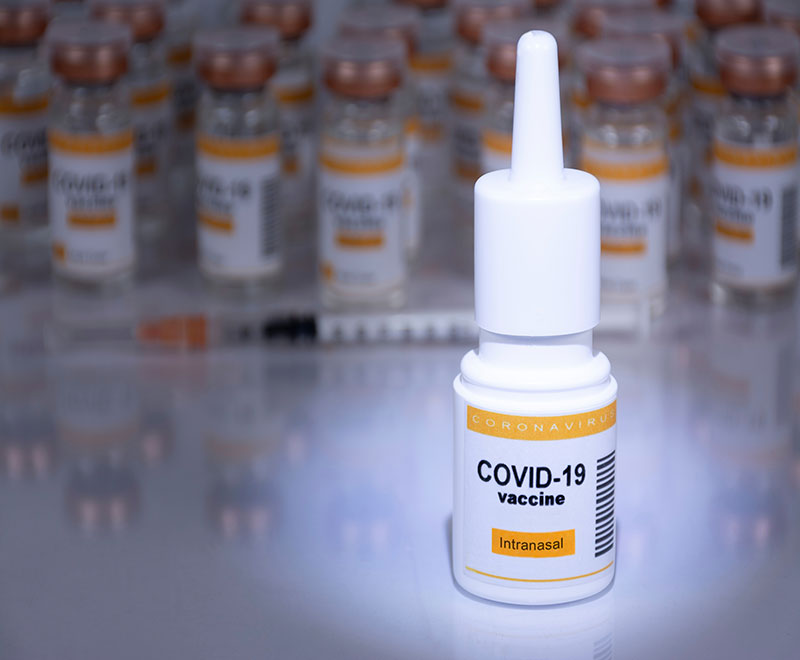
A study by researchers at Washington University School of Medicine in St. Louis indicates next-generation vaccines that target a virus’s points of entry — the nose and mouth — may be able to contain the spread of respiratory infections and prevent transmission.
New Test Can Accurately Predict 90 Percent of Alzheimer’s Cases

A new study has found a combined blood test for cognitive decline has a 90 percent accuracy rate in determining whether memory loss is due to Alzheimer’s disease.
COVID Vaccines: What’s Available Now and What’s in the Works?
Three top vaccine producers are making newer vaccines that not only protect against the newer sublineages of COVID-19, but that also protect against other respiratory viruses.
How FDA Is Working to Accelerate Rare Disease Treatments

Through regulatory pathways and patient engagement, FDA is helping to advance treatment innovations for rare diseases.
Cryoprecipitate, Fibrinogen Concentrates and New Pathogen Reduced Cryo Product Vie for Use in Massive Hemorrhage

Does the shorter preparation time for fibrinogen concentrates make them a reasonable option in lieu of IFC in defined patient populations experiencing massive hemorrhage?