FDA Expands Indication for Octapharma’s WILATE to Hemophilia A

The U.S. Food and Drug Administration (FDA) has approved WILATE for treatment of adults and adolescents with hemophilia A for routine prophylaxis.
Medicare Will Now Cover Acupuncture for Chronic Low Back Pain
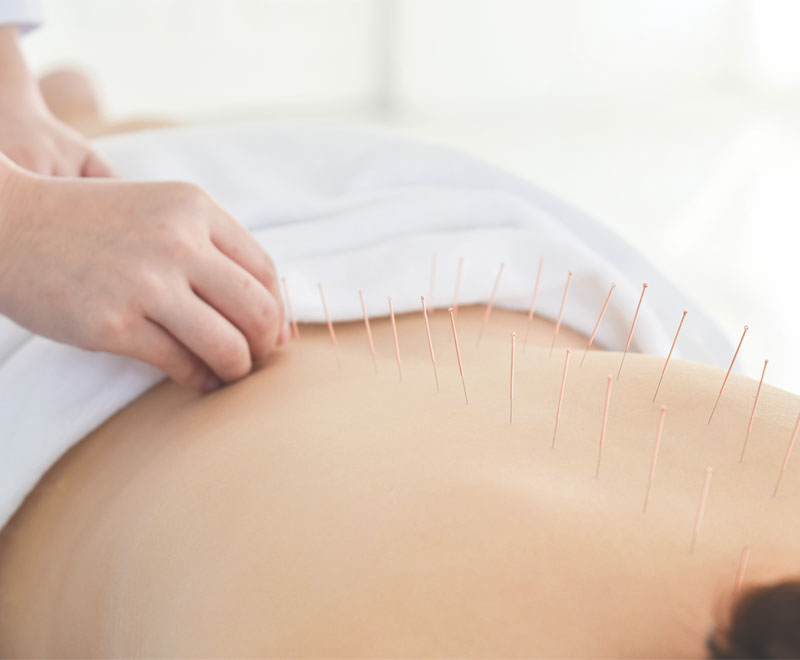
The Centers for Medicare and Medicaid Services (CMS) finalized a decision to cover acupuncture for Medicare patients with chronic low back pain.
Plasma-Derived ApoA-1
Findings suggest CSL112 can boost cholesterol efflux capacity in patients with impaired endogenous HDL function. Encouragingly, several studies have documented its ability to reduce atherosclerotic plaque volume.
Nutrigenomics: How Genes and Nutrition Interact

Using nutrition to benefit health through the care and feeding of genes, though still in its infancy, is an exciting field of study seeing exponential growth.
SCIG with Recombinant Human Hyaluronidase Is Safe and Preferred vs IVIG by Some Multifocal Motor Neuropathy Patients
A team of Dutch investigators enrolled 18 multifocal motor neuropathy (MMN) patients on intravenous immune globulin(IVIG) treatment in a prospective open-label study to evaluate the comparative safety of treatment with 10% human immune globulin whose subcutaneous administration is facilitated with recombinant human hyaluronidase (fSCIG) (HyQvia).
Excellent Response to Therapeutic Plasma Exchange in Myasthenia Gravis Patients with or Without Autoantibodies

A study out of the University of Texas Southwestern Medical Center found a 96 percent response rate to treatment with therapeutic plasma exchange (PLEX) in a series of 58 consecutive myasthenia gravis (MG) patients, with no significant difference in response between those with or without autoantibodies.
Novo Nordisk’s Esperoct Approved to Treat Individuals with Hemophilia A

FDA approved Esperoct (antihemophilic factor[recombinant], glycopegylatedexei), an extended half-life factor VIII molecule for replacement therapy in people with hemophilia A.
First Therapy Approved to Treat Rare Blood Clotting Disorder
FDA has approved Cablivi (caplacizumab-yhdp) injection, the first therapy specifically indicated, in combination with plasma exchange and immunosuppressive
therapy, for the treatment of adult patients with acquired thrombotic thrombocytopenic purpura.
FDA Approves Hepatitis A and Measles Exposure Drug

FDA has approved Grifols’ GamaSTAN to treat people exposed to measles and the hepatitis A viruses.
Subcutaneous Lanadelumab Treatment Reduces Attack Rate in Patients with Hereditary Angioedema
Subcutaneous administration of lanadelumab, an investigational monoclonal antibody intended for the prevention of attacks in patients with type I or II hereditary angioedema (HAE), significantly reduced the mean number of attacks compared to placebo treatment.