Industry News
Research, Science & Manufacturer Updates
Washington Report Articles
The U.S. Department of Health and Human Services has awarded $94 million in Affordable Care Act funding to 271 health centers in 45 states, the District of Columbia and Puerto Rico.
The Centers for Medicare and Medicaid Services announced 10 states in four regions in which it will launch its primary care quality improvement initiative.
The National Institutes of Health will spend $260 million over four years to fund four genome sequencing and analysis centers whose research is expected to focus on understanding the genomic bases of common and rare human diseases.
Three health centers in America Samoa and the U.S. Virgin Islands and their 12 delivery sites that served nearly 26,000 patients in 2014, including more than 6,000 women ages 15 years to 45 years, have been awarded $742,000 in funding to fight the Zika virus.
The U.S. Senate approved the Medicare “lock-in” provision that gives Medicare Part D plans the authority to require at-risk beneficiaries to use a single prescriber and pharmacy for frequently abused drugs.
The Centers for Medicare and Medicaid Services will implement a new primary care payment model affecting up to 5,000 practices and more than than 20,000 clinicians.
The Centers for Medicare and Medicaid Services issued a final rule mandating prior authorization for durable medical equipment.
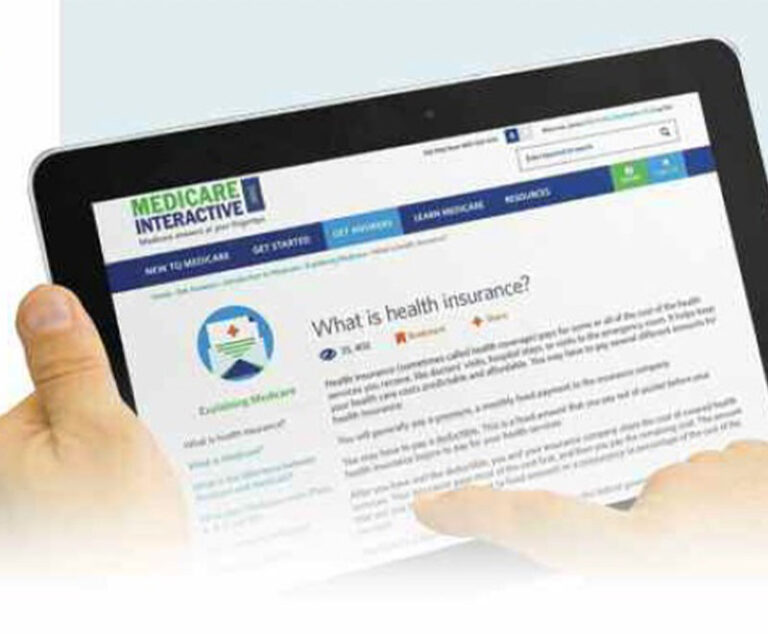
The Medicare Rights Center has launched Medicare Interactive (MI), a free online resource with hundreds of answers to Medicare questions.
The Centers for Medicare and Medicaid Services published its annual Notice of Benefit and Payment Parameters, which governs participation in the Affordable Care Act health insurance marketplaces for 2017.
The Centers for Medicare and Medicaid Services has published several policies on biosimilars reimbursement.
In response to concerns by providers about the ICD-9 to ICD-10 coding system, the Centers for Medicare and Medicaid Services says it will reimburse for incorrectly coded claims for one year past the Oct. 1, 2015, deadline.
The Centers for Medicare and Medicaid Services has created an interactive online dashboard to allow the public and policymakers to explore the financial burden that high-expense drugs place on the Medicare program and the nation’s seniors.