Industry News
Research, Science & Manufacturer Updates
Washington Report Articles
The U.S. Department of Health and Human Services (HHS) has announced additional funding under the Affordable Care Act (ACA) and other programs. Under the ACA, nearly $500 million is being awarded to health centers nationwide to provide primary care services to those who need them most.
Doctors who care for tens of millions of chronically ill Medicare patients are failing to take advantage of federal dollars intended to improve care and reduce hospital readmissions and overall costs, according to the Centers for Medicare and Medicaid Services (CMS).
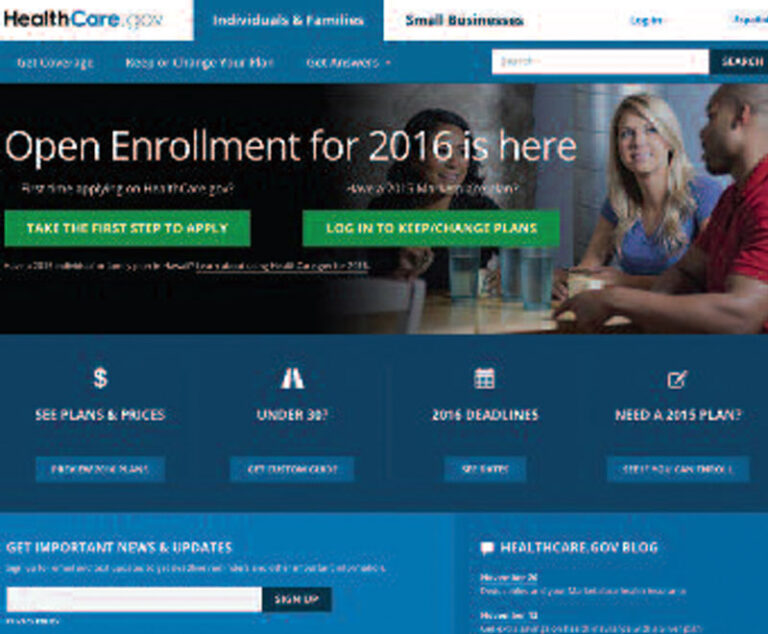
Healthcare.gov, the main entry point for people seeking insurance in 38 states served by the federal exchange, features three new window-shopping features.
To make the U.S.healthcare system more accountable, the federal government continues to release Medicare claims and payment information.
The U.S. Senate Appropriations Committee approved the fiscal year 2016 appropriations bill to fund the U.S. Food and Drug Administration but at levels far below what President Obama requested.
President Obama signed a bill into law that will compensate patients for participating in clinical studies of rare diseases.
The Medicare Access and CHIP Reauthorization Act of 2015, which was enacted into law on April 16, will prohibit Medicare supplemental insurance(Medigap) policies from covering the Part B deductible for people who become eligible for Medicare on or afterJan. 1, 2020.
The U.S. FDA has issued a final rule requiring manufacturers to give six months’ notice if they plan to discontinue or interrupt production.
A final rule issued by the Centers for Medicare and Medicaid Services is intended to maintain the rigor of the Medicare Shared Savings program (created by the Affordable Care Act),while ensuring providers continue to participate.
Nationwide, nearly 11.7 million consumers selected or were automatically re-enrolled in health insurance coverage through the Health Insurance Marketplace as of Feb. 22, according to a report by the U.S. Department of Health and Human Services.
The Medicare Rights Center has released a new Medicare Snapshot: Stories from the Helpline that addresses Medicare enrollment pitfalls and outlines needed improvements.
The U.S. Department of Health and Human Services’ Office of the Assistant Secretary for Preparedness and Response (ASPR) has awarded approximately $12 million to BioCryst Pharmaceuticals for the advanced development of a promising experimental drug for Ebola.