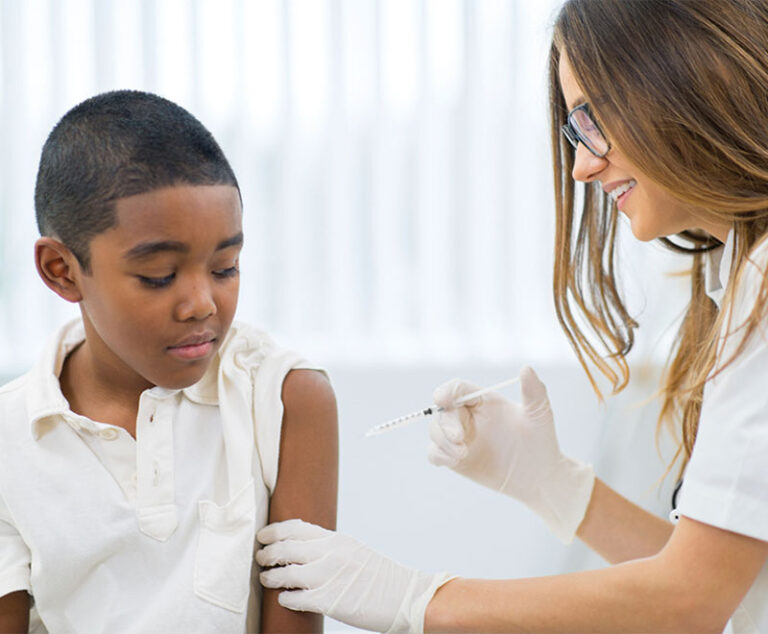
Industry News
Research, Science & Manufacturer Updates
Vaccines Articles
Researchers found that flu vaccines are more effective when given in the morning because patients’ immune systems are capable of producing more antibodies in response to the vaccine in the first part of the day.
A new tobacco-based seasonal influenza vaccine being developed by Mitsubishi Tanabe Pharma and currently in Phase III studies could potentially rival traditional chicken egg-based vaccines.
Findings from a first in-human study for a new malaria vaccine candidate have shown a robust immune response while significantly delaying parasitemia.
Preliminary overall 2015-16 influenza vaccine effectiveness was 59 percent, according to the Centers for Disease Control and Prevention.
A new form of hybridized sound waves developed by Australian researchers may allow drugs and vaccines to be delivered to the body through a nebulizer in a fine mist inhaled into the lungs.
Two studies presented at the International Conference on Emerging Infectious Diseases show that the influenza vaccine can protect for six months, last throughout the flu season and reduce hospitalization in children.
The U.S. Food and Drug Administration(FDA) has granted exclusivity to Flublok influenza vaccine for a period of 12 years.
A new study shows that people who receive a shingles vaccine but still contract shingles have a lower risk of developing post-herpatic neuralgia (PHN).
In a first-in-people clinical trial, personalized tailor-made melanoma vaccines given to three patients with advanced melanoma appeared to increase the number and diversity of cancer-fighting T cells responding to the tumors.
At its annual meeting in June, the American Medical Association (AMA) adopted a new policy to seek more stringent state immunization requirements to allow exemptions only for medical reasons.
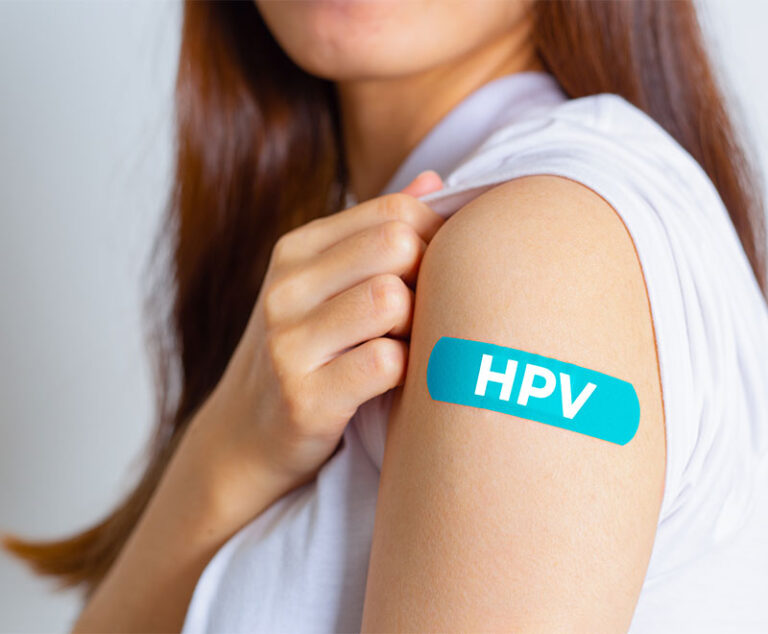
Three recent studies have found that interventions increase the rates of human papillomavirus (HPV) vaccination among teens and young women.
A new simulation study that evaluated the relationship between Guillain-Barré syndrome (GBS) risk and influenza vaccine and illness suggests that the vaccine reduces the risk for GBS.