Industry News
Research, Science & Manufacturer Updates
Vaccines Articles
Researchers at the University of Oxford in the United Kingdom have linked several genetic variations with the level of protective antibodies generated following routine childhood immunizations.
Sanofi and Merck’s Vaxelis has been approved by the U.S. Food and Drug Administration.
A new study by researchers at Columbia University has found handing a pamphlet about influenza (flu) to parents in pediatricians’ waiting rooms can have a significant impact on increasing the uptake of the flu vaccine.
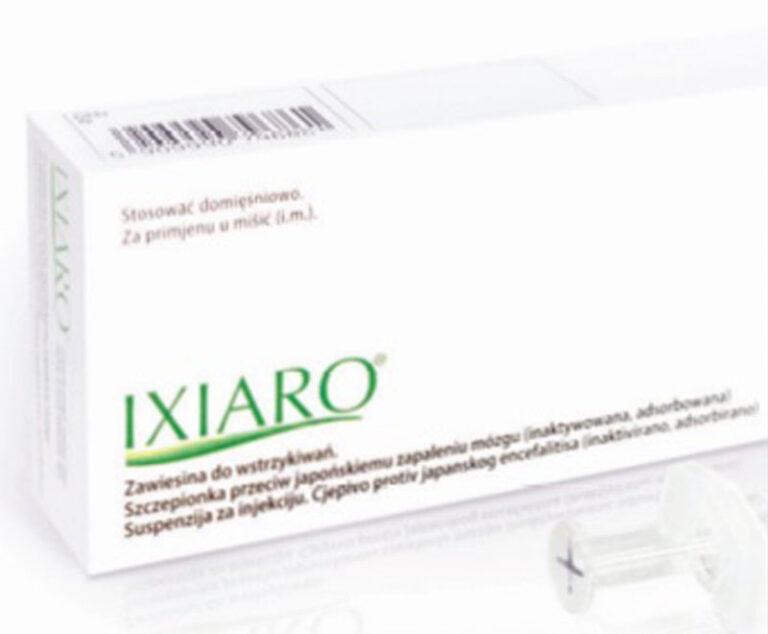
Valneva USA has received FDA approval of an accelerated dosing regimen for IXIARO (Japanese encephalitis vaccine, inactivated, adsorbed).
A new study conducted by investigators at the Center for Clinical Epidemiology and Population Health at the Marshfield Clinic Research Institute in Wisconsin has found the flu vaccine does not cause miscarriages in pregnant women.
The WHO advisory board issued its new recommendations on the composition of the influenza vaccines for use in the 2019-20 flu season in the Northern Hemisphere.
Although more antivaccination legislation was introduced between 2011 and 2017, bills that limited vaccine exemptions were significantly more likely to be enacted.
A recent meta-analysis of pooled individual patient-level data, researchers found reduced effectiveness of the quadrivalent live-attenuated influenza vaccine (LAIV4) against influenza A/H1N1pdm09 compared with inactivated influenza vaccine (IIV) in children and adolescents.
The Human Vaccine Project aims to understand how the human immune system can develop longer-lasting protection against influenza.
FDA granted Sanofi Pasteur’s Adacel Tdap absorbed vaccine expanded indication to include repeat vaccinations for tetanus, diphtheria and pertussis.
A new survey shows more than 40 percent of Americans haven’t been vaccinated against the flu and aren’t planning to be due to misconceptions.
A new study shows pregnant women hospitalized in the ICU with flu are four times more likely to deliver prematurely and four-and-a-half times more likely to have a baby of low birth weight.