Industry News
Research, Science & Manufacturer Updates
Medicines Articles
The U.S. Food and Drug Administration has launched the Secure Supply Chain Pilot Program (SSCPP) to enable qualified firms to expedite the importation of active pharmaceutical ingredients and finished drugs into the United States.
The U.S. Food and Drug Administration has instructed manufacturers to add information to the current boxed warning in the labeling of all intravenous immune globulin (IVIG) products, and add a similar standardized boxed warning for all subcutaneous and intramuscular IG products.
The U.S. Food and Drug Administration(FDA) has approved an expanded indication for CSL Behring’s Corifact, factor XIII (FXIII) concentrate (human) to include the perioperative management of surgical bleeding in adult and pediatric patients with congenital FXIII deficiency.
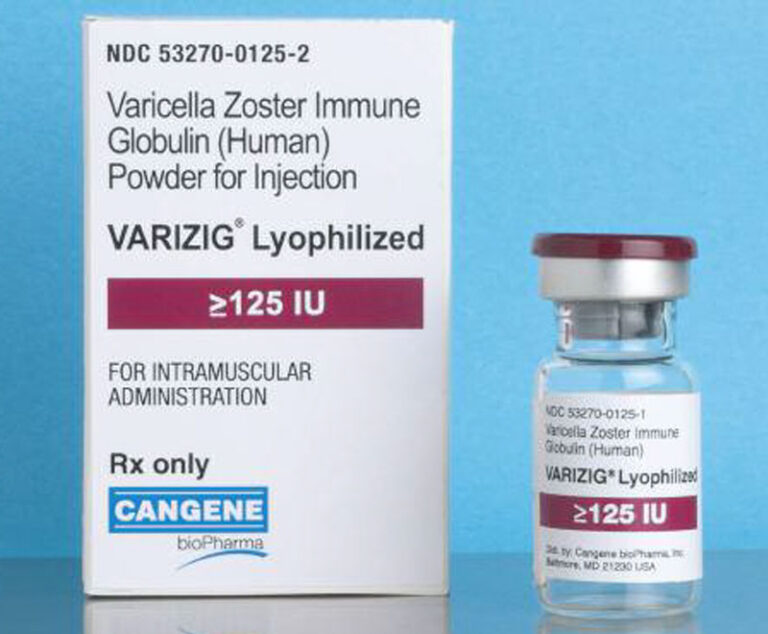
VARIZIG, a hyperimmune globulin indicated for post-exposure prophylaxis of varicella zoster virus (VZV) in high-risk patients, has been approved by the U.S.Food and Drug Administration.
Octapharma USA’s Octaplas, a solvent/detergent-treated pooled human plasma, has been approved by the U.S. Food and Drug Administration.
Octapharma USA has started an initiative to make octagam (immune globulin intravenous [human] 5%), a therapy for primary immune deficiency, widely available to covered entities in the 340B Drug Pricing Program.
Baxter International has received U.S. Food and Drug Administration approval for a 4,000 IU dosage of Advate (Antihemophilic Factor [Recombinant], Plasma/Albumin-Free Method).
CSL Behring has been granted orphan drug designation by the U.S.Food and Drug Administration for its novel recombinant fusion protein linking coagulation factor VIIa with albumin (rVIIa-FP).
Recent studies suggest that the divergent outcomes in Alzheimer’s disease clinical studies of intravenous immune globulin (IVIG) may be due to differences in temporal administration and administered dosages.
Cangene Corp. has issued a voluntary recall of several finished product lots associated with one bulk lot of Hepatitis B Immune Globulin ([Human] HepaGam B > 312 IU/mL).
The U.S. Food and Drug Administration approved Baxter International Inc.’s Advate (antihemophilic factor [recombinant] plasma/albumin free method) for hemophilia A.
The U.S. Food and Drug Administration and the Committee for Medicinal Products for Human Use in Europe have approved the return of Octagam 5% (human normal immunoglobulin 50 mg/ml) to the market. Marketing authorization was suspended in August 2010 in the U.S.