Industry News
Research, Science & Manufacturer Updates
Medicines Articles
A new report shows that stem cell transplants might soon offer multiple sclerosis (MS) patients an effective way to stave off relapses and improve their overall neurologic condition.
Scientists at the University of South Australia and colleagues from Third Military Medical University in Chongqing, China, have found that the drug Edaravone alleviates Alzheimer’s disease pathologies at multiple levels and improves learning and memory functions in mice.
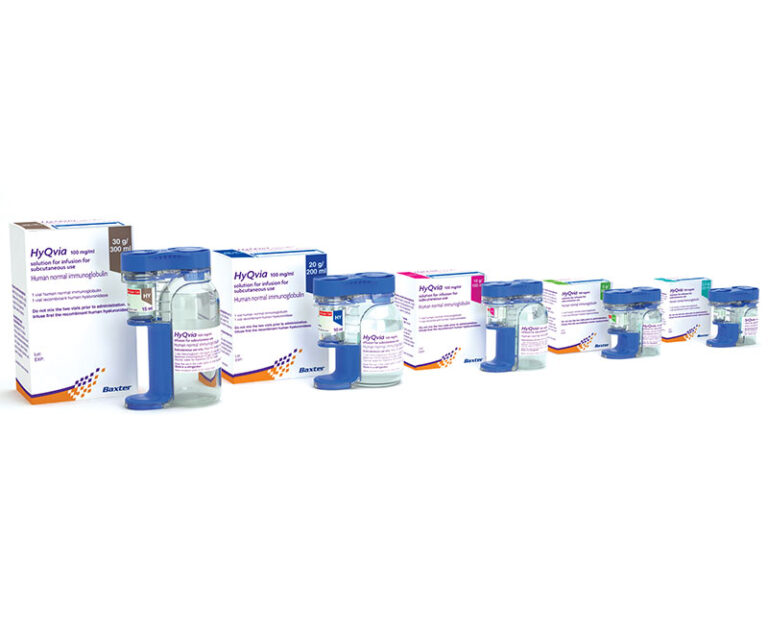
The Centers for Medicare and Medicaid Services (CMS) has expanded coverage to include in-home use of HYQVIA(immune globulin infusion 10 percent [human] with recombinant humanhyaluronidase) to treat primary immunodeficiency patients.
Pfizer (a licensee of Gliknik Inc.) has been granted orphan drug designation by the U.S. Food and Drug Administration (FDA) for its recombinant intravenous immune globulin (IVIG)-mimetic drug
GL-2045 to treat chronic inflammatory demyelinating polyneuropathy (CIDP).
CSL Behring’s Biologics License Application for the marketing authorization of its long-acting fusion protein linking recombinant coagulation factor IX with recombinant albumin, rIX-FP, has been accepted for review by the U.S.
Octapharma USA has launched the Octapharma Co-Pay Assistance Program available to von Willebrand’s disease patients who are currently receiving Wilate or have a prescription to begin therapy.
The U.S. Department of Health and Human Services’ Office of the Assistant Secretary for Preparedness and Response (ASPR) has awarded approximately $12 million to BioCryst Pharmaceuticals for the advanced development of a promising experimental drug for Ebola.
Novo Nordisk has launched Novoeight (antihemophilic factor [recombinant]) in the U.S. for people living with hemophilia A.
A new experimental drug could boost the immune system in older adults by as much as 20 percent, as well as help them to delay other aging effects.
Octapharma USA has launched the Octapharma Co-Pay AssistanceProgram available to von Willebrand’s disease patients who are currently receiving Wilate (von Willebrand factor/coagulation factor VIII complex[human]) or have a prescription to begin therapy.
The U.S. Food and Drug Administration(FDA) has approved Octapharma’s manufacturing facility in Vienna, Austria, for the production of Octagam 10% (immune globulin intravenous [human] 10% [100mg/mL] liquid preparation).
In June, the U.S. Supreme Court voted 5-4 to allow a key exemption to the Affordable Care Act’s contraception coverage requirements, meaning closely held, for-profit businesses can assert a religious objection.