Industry News
Research, Science & Manufacturer Updates
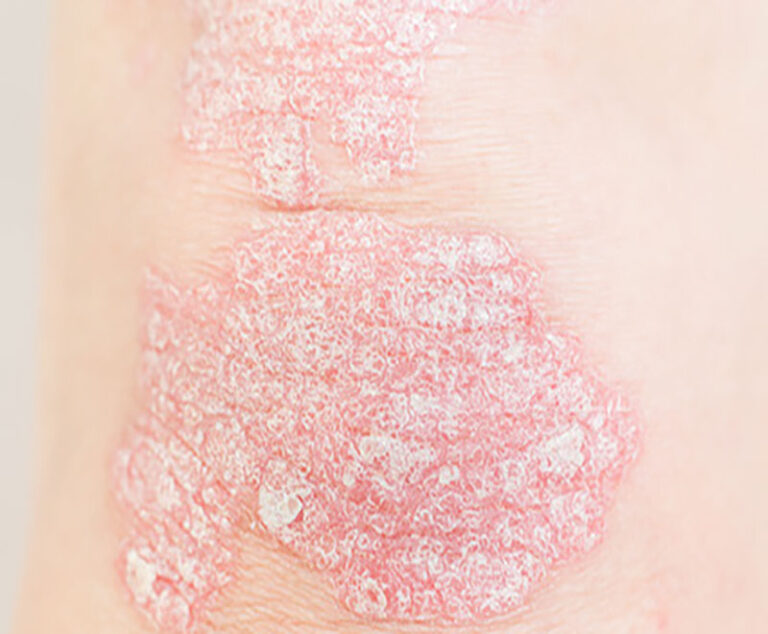
The U.S. Food and Drug Admini-stration has approved Selarsdi (ustekinumab-aekn) injection for subcutaneous use, as a biosimilar to Stelara, for the treatment of moderate to severe plaque psoriasis and for active psoriatic arthritis in adults and pediatric patients 6 years and older.
Researchers at the University of California, San Francisco, have identified a specific pattern of autoantibodies in the blood that precedes the clinical onset of multiple sclerosis.
The U.S. Food and Drug Administration (FDA) has granted orphan drug exclusivity for Octapharma’s wilate, von Willebrand factor/coagulation factor VIII complex (human) lyophilized powder for solution for intravenous injection, for routine prophylaxis to reduce the frequency of bleeding episodes in adults and children 6 years of age and older with von Willebrand disease (VWD).
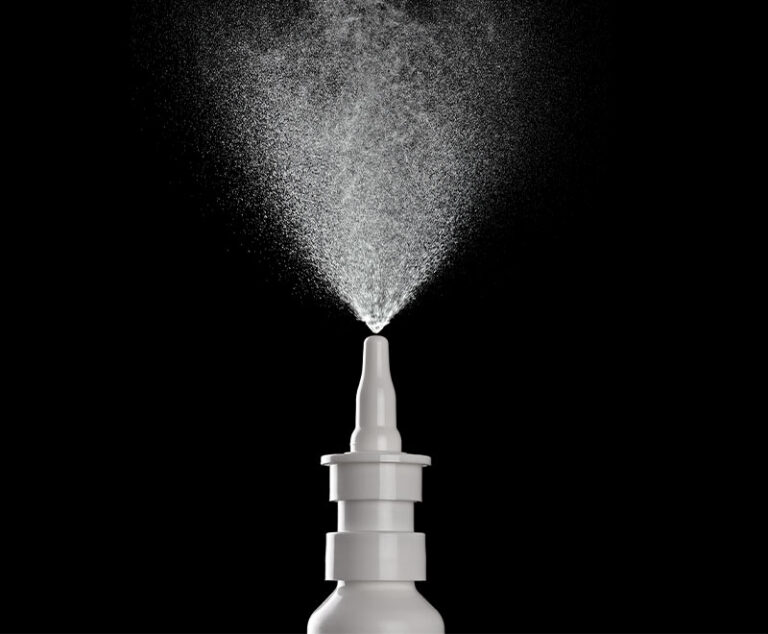
A Phase I clinical trial testing the safety of an experimental nasal vaccine, MPV/S-2P, that may provide enhanced breadth of protection against emerging variants of SARS-CoV-2, the virus that causes COVID-19, is now enrolling healthy adults at three sites in the United States.
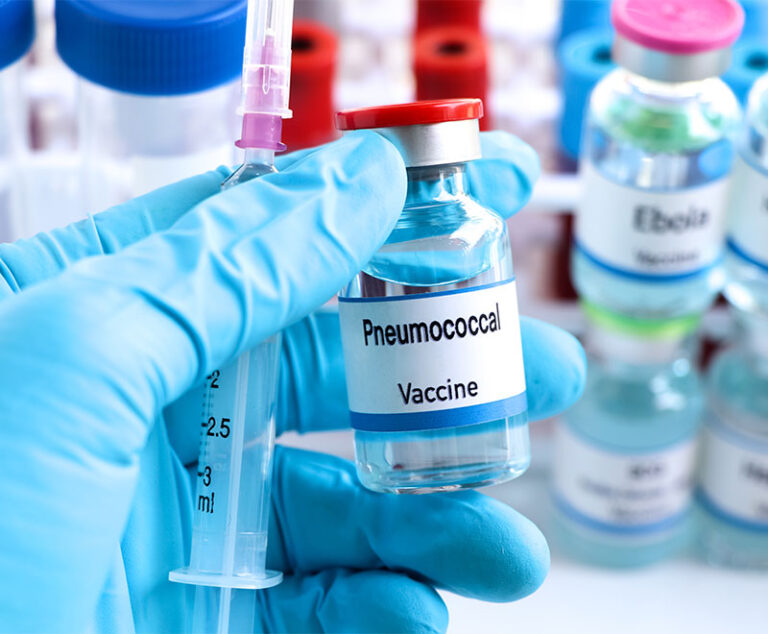
The U.S. Food and Drug Administration has approved CAPVAXIVE for active immunization for the prevention of invasive disease caused by Streptococcus pneumoniae in individuals 18 years of age and older.
Biotest has received approval from the U.S. Food and Drug Administration for Yimmug to treat primary immunodeficiencies in patients 2 years and older.
CMS finalized the CMS Interoperability and Prior Authorization Final Rule to improve the electronic exchange of health information and prior authorization processes for medical items and services.
Investigators at the Bern University Hospital conducted a single-center randomized clinical trial to quantify and compare the PVE properties of iso-oncotic 5% albumin, hyper-oncotic 20% albumin and Ringer’s lactate (RL) in patients undergoing radical cystectomy.
The Phase IIIb study of emicizumab shows promise for reducing risk of spontaneous and traumatic bleeds in infants with hemophilia A.
Researchers have discovered a rare type of immune cell that may predict how likely some patients with skin cancer will respond to immunotherapy treatment.
Researchers at City of Hope have discovered that a type of immune cell in the human body known to be important for allergy and other immune responses can also attack cancer.
A new vaccine may help speed up the process of making antibodies against SARS-CoV-2 by using preexisting immunity to a separate virus (the influenza virus).