Industry News
Research, Science & Manufacturer Updates
Immune Globulin Articles
Johnson & Johnson’s anti-FcRn antibody nipocalimab is the first investigational therapy to be granted breakthrough therapy designation by the U.S. Food and Drug Administration as a treatment for adults with moderate-to-severe Sjögren’s disease.
A new study indicates intravenous immun globulin (IVIG) therapy can reduce symptom severity for patients with autoimmune gastrointestinal dysmotility (AGID), also known as gastroparesis.
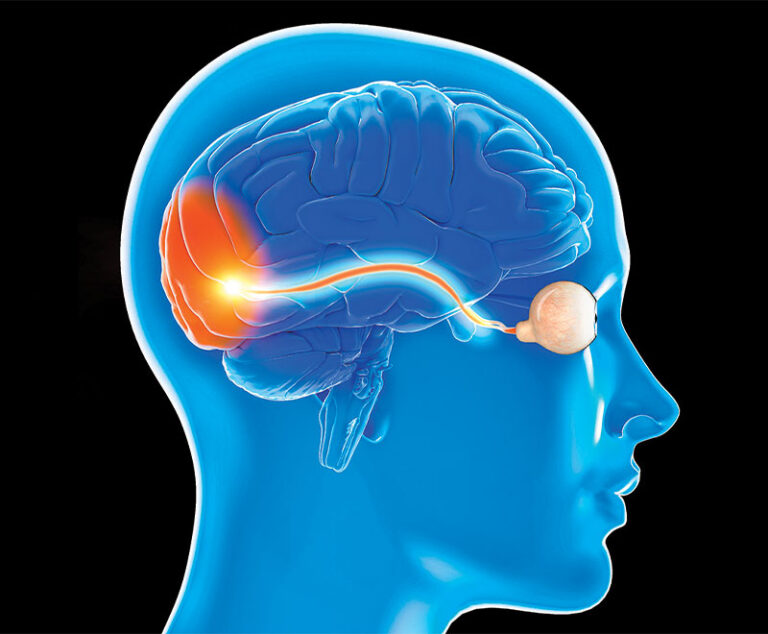
A meta-analysis and systematic review compared the efficacy of corticosteroids (CS) alone against CS combined with intravenous immune globulin (IVIG) or CS combined with plasmapheresis (PP) in patients with steroid-resistant optic neuritis.
The U.S. Food and Drug Administration (FDA) has approved an expanded label for Grifols’ XEMBIFY (20% subcutaneous immune globulin [SCIG]) to include treatment-naïve patients with primary humoral immunodeficiencies (PI).
Samsung Bioepis’ biologics license application for Epysqli (eculizumab-aagh) as a biosimilar to Soliris (eculizumab) has been approved by the U.S. Food and Drug Administration (FDA) to treat patients with paroxysmal nocturnal hemoglobinuria (PNH) to reduce hemolysis and atypical hemolytic uremic syndrome (aHUS) to inhibit complement-mediated thrombotic microangiopathy.
Biotest has received approval from the U.S. Food and Drug Administration for Yimmug to treat primary immunodeficiencies in patients 2 years and older.
Data suggests the bispecific BCMA-targeted antibody teclistamab may lead to grade 3 to 5 infections in as many as 44.8 percent of multiple myeloma patients, and hypogammaglobulinemia was noted in nearly 75 percent of patients.
The combination of intravenous immune globulin (IVIG) and corticosteroids is a more efficient and rapid treatment for relapsed immune thrombocytopenic purpura (ITP) in adults compared with the use of either therapy alone.
Recent case series findings, as well as previous studies, show that children with multisystem inflammatory syndrome (MIS-C) who present with signs of active neurological symptoms may show improvement with intravenous immune globulin (IVIG) and corticosteroids.
A recent study has found that one specific intravenous immune globulin (IVIG) product was more effective than others in counteracting the cognitive deficits, ameliorating b-amyloid (Ab) deposits and tau phosphorylation among mice with Alzheimer disease (AD)
New recommendations from the American Association of Neuromuscular and Electrodiagnostic Medicine advise against using IVIG in some cases.
A systematic search of leading databases and registries by Chinese collaborators compared the efficacy of IVIG to routine care for hospitalized COVID-19 patients.