Industry News
Research, Science & Manufacturer Updates
Immune Globulin Articles
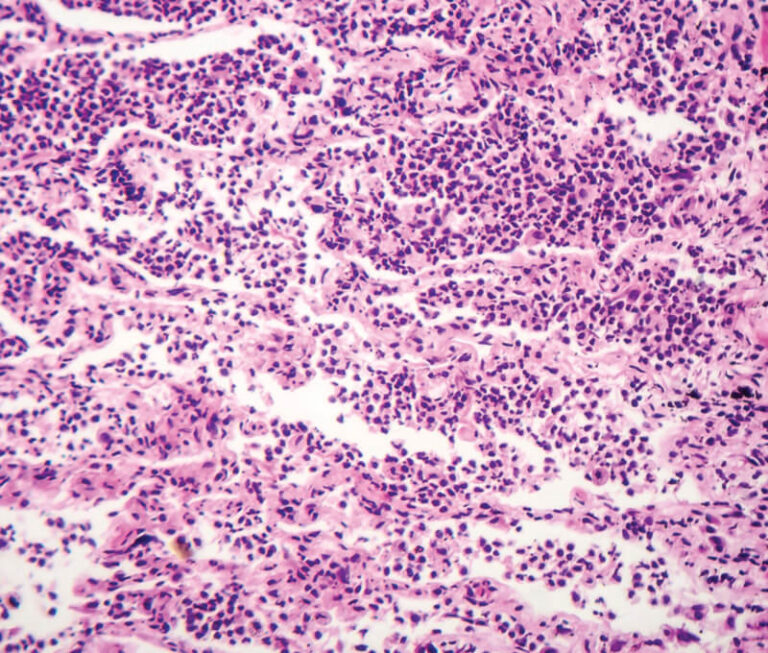
Investigators have found that intravenous immune globulin may have a positive effect on acute exacerbation of fibrotic idiopathic interstitial pneumonias.
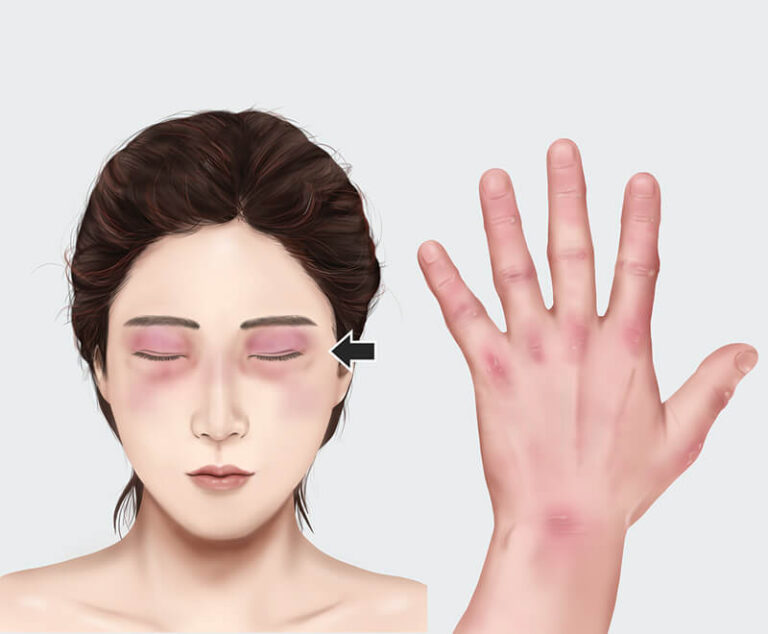
A study evaluating intravenous immune globulin for the treatment of dermatomyositis has found it significantly improved patient outcomes.
Dutch investigators conducted a systematic review and meta-analysis of studies on the effectiveness of IVIG treatment of this specific population.
A new study shows treatment with IVIG shows promise for children and young adults with Down syndrome regression disorder (DSRD).
Italian investigators conducted a trial to assess the safety and efficacy of IVIG in treatment-resistant painful diabetic polyneuropathy (DPN).
A new systematic review and meta-analysis conducted by U.S. and Nepalese collaborators supports the use of IVIG with glucocorticoids compared to IVIG alone.
While intravenous immune globulin (IVIG) therapy is efficacious for patients with chronic inflammatory demyelinating polyneuropathy (CIDP), the lack of biomarkers for disease activity makes the need for ongoing treatment difficult to assess.
Observational studies suggest immune globulin (IG) treatment may reduce the frequency of acute exacerbations of chronic obstructive pulmonary disease (AECOPD).
A large multicenter clinical trial has found intravenous immune globulin (IVIG) plus glucocorticoids may be better than IVIG alone for treating multisystem inflammatory syndrome in children (MIS-C) caused by COVID-19.
Sandoz has announced that the citrate-free high-concentration formulation of its biosimilar Hyrimoz (adalimumab-adaz) injection became available in the United States starting July 1, 2023.
The U.S. Food and Drug Administration (FDA) has approved Ziextenzo (pegfilgrastim-bmez), the 24th biosimilar approval in the U.S.
Octapharma introduced its newest product, Cutaquig (immune globulin subcutaneous [human] 16.5% solution) at the Immune Deficiency Foundation (IDF) National Conference in June.