Industry News
Research, Science & Manufacturer Updates
Immune Globulin Articles
A trial was conducted to assess the safety and efficacy of hIVIG (in conjunction with standard care) in adults hospitalized with laboratory-confirmed influenza A or B infection.
The U.S. Patent and Trademark Office has issued a patent to ADMA Biologics for methods of treatment and prevention of S. pneumonia infection.
A team of Dutch investigators enrolled 18 multifocal motor neuropathy (MMN) patients on intravenous immune globulin(IVIG) treatment in a prospective open-label study to evaluate the comparative safety of treatment with 10% human immune globulin whose subcutaneous administration is facilitated with recombinant human hyaluronidase (fSCIG) (HyQvia).
Canadian investigators at Ottawa Hospital have reported a very high success rate with a nurse-led individualized program to facilitate a smooth transition in patients with neurological disorders from chronic intravenous immune globulin (IVIG) to subcutaneous immune globulin (SCIG) treatment.
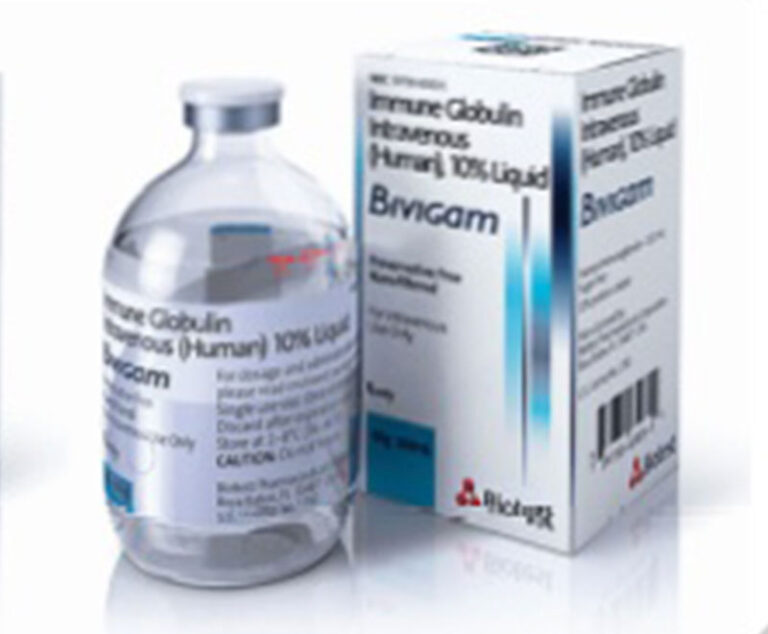
ADMA Biologics has received approval from FDA for its prior approval supplement for Bivigam, allowing the company to use its optimized IVIG manufacturing process and market Bivigam to PI patients in the U.S.
Investigators at the Huazhong University of Science and Technology conducted a meta-analysis of published clinical trials that evaluated IVIG therapy in acute myocarditis.
Investigators from the Belgian biotechnology company argenx and U.S. collaborators conducted a PhaseI clinical study to assess a novel modified antibody Fc fragment (efgartigimod) that reduces the circulating IgG level by blocking neonatal Fc receptor-mediated IgG recycling.
In the largest case series of children with autism spectrum disorder (ASD) treated with IVIG, researchers identified brain-targeted autoantibodies in children with ASD.
A pilot study conducted by U.S. investigators observed improvements in cognitive and behavioral function in 14 children with autism spectrum disorder and evidence of immune dysfunction who were administered high-dose intravenous immune globulin (IVIG) treatment over a period of 30 weeks.
Grifols’ higher-potency rabies immune globulin (RIG), HyperRAB S/D, was made available to healthcare providers
Discontinuation of the product is due to the preference among healthcare professionals and patients for newer, more advanced immune globulin options.
This first-ever prospective economic analysis by Canadian investigators found that home-based subcutaneous immune globulin (SCIG) therapy was associated with significantly lower average total nondrug costs than hospital-based intravenous immune globulin (IVIG) therapy for patients with primary immunodeficiency disorders.