Industry News
Research, Science & Manufacturer Updates
The U.S. Department of Health and Human Services has founded the Office of Long COVID Research and Practice to lead the Long COVID response and coordination across the federal government. In addition, the National Institutes of Health launched Long COVID clinical trials through the RECOVER Initiative.
The U.S. Department of Health and Human services has awarded more than $147 million to 49 recipients to advance the Ending the HIV Epidemic in the U.S. initiative, which is part of the ongoing efforts to reduce the number of new infections in the United States by at least 90 percent by 2030.
Through the Health Resources and Services Administration, the U.S. Department of Health and Human Services has announced the availability of approximately $25 million to expand primary healthcare, including mental health services, in schools.
The Guiding an Improved Dementia Experience (GUIDE) Model, which aims to improve the quality of life for people living with dementia, reduce strain on unpaid caregivers, and help people remain in their homes and communities through a package of care coordination and management, caregiving education and support, and respite services has been established by the U.S. Department of Health and Human Services.
An expanded indication for ERVECO for the prevention of disease caused by Zaire ebolavirus in individuals 12 months of age and older has been approved by the U.S. Food and Drug Administration.
A recent study has found chronic immune-related side effects are common in patients with skin cancer who are treated with postsurgical Opdivo (nivolumab) or Keytruda (pembrolizumab), although for some individuals, these toxicities resolve by the 18-month mark.
Recent case series findings, as well as previous studies, show that children with multisystem inflammatory syndrome (MIS-C) who present with signs of active neurological symptoms may show improvement with intravenous immune globulin (IVIG) and corticosteroids.
A new study conducted at the University of Texas Health Science Center at Houston found that people who received shingles and pneumonia vaccines — along with tetanus and diphtheria — had as much as a 30 percent reduced risk of developing Alzheimer's the most common type of dementia.
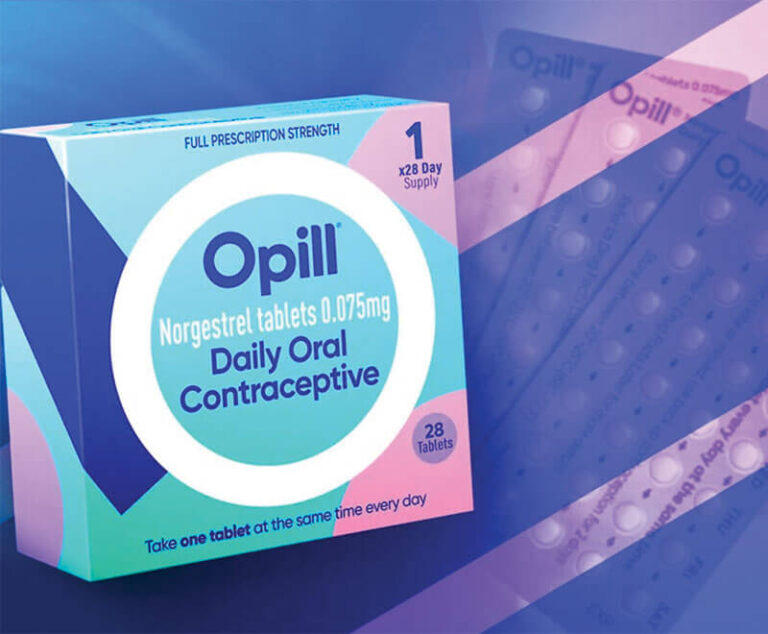
The U.S. Food and Drug Administration has approved the birth control pill Opill (norgestrel), manufactured by Perrigo, to be available over-the-counter — the first nonprescription birth control pill in the United States.
Pfizer's ABRYSVO, a respiratory syncytial virus (RSV) vaccine, has been approved by the U.S. Food and Drug Administration for the prevention of lower respiratory tract disease (LRTD) and severe LRTD caused by RSV in infants from birth up to 6 months of age by active immunization of pregnant women at 32 through 36 weeks gestational age.
The U.S. Food and Drug Administration (FDA) has granted 510(k) clearance to Precision BioLogic’s CRYOcheck Factor VIII (FVIII) Deficient Plasma with von Willebrand factor (VWF), opening the pathway for the company to launch the product in the U.S.
Researchers from the Pritzker School of Molecular Engineering at the University of Chicago have developed a new type of vaccine called an “inverse vaccine that completely reversed autoimmune diseases without fully shutting down the rest of the immune system.