Industry News
Research, Science & Manufacturer Updates
FDA has approved Cablivi (caplacizumab-yhdp) injection, the first therapy specifically indicated, in combination with plasma exchange and immunosuppressive
therapy, for the treatment of adult patients with acquired thrombotic thrombocytopenic purpura.
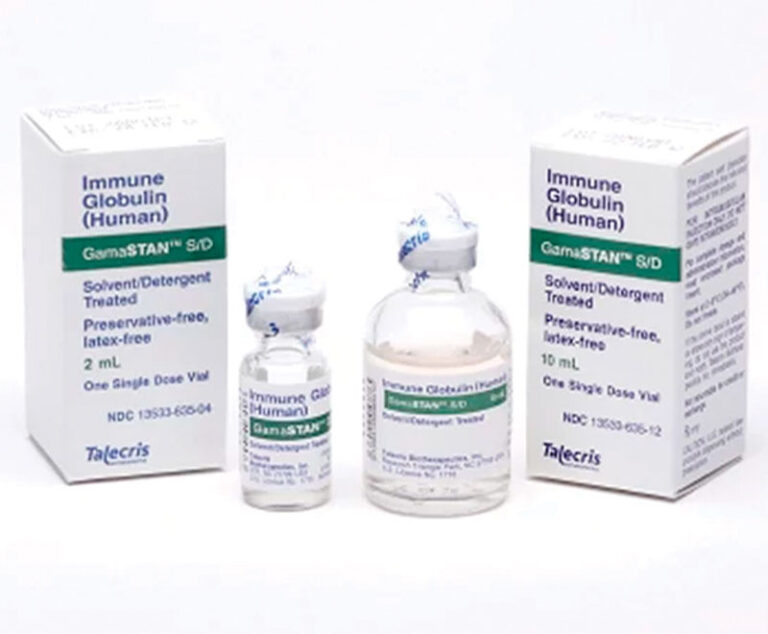
FDA has approved Grifols’ GamaSTAN to treat people exposed to measles and the hepatitis A viruses.
Although more antivaccination legislation was introduced between 2011 and 2017, bills that limited vaccine exemptions were significantly more likely to be enacted.
Subcutaneous administration of lanadelumab, an investigational monoclonal antibody intended for the prevention of attacks in patients with type I or II hereditary angioedema (HAE), significantly reduced the mean number of attacks compared to placebo treatment.
A recent meta-analysis of pooled individual patient-level data, researchers found reduced effectiveness of the quadrivalent live-attenuated influenza vaccine (LAIV4) against influenza A/H1N1pdm09 compared with inactivated influenza vaccine (IIV) in children and adolescents.
Investigators at the Huazhong University of Science and Technology conducted a meta-analysis of published clinical trials that evaluated IVIG therapy in acute myocarditis.
FDA has approved Amgen’s romiplostim to treat pediatric patients ages 1 year and older with immune thrombocytopenia for a minimum of six months and who have had an insufficient response to corticosteroids, immune globulin or splenectomy.
The Human Vaccine Project aims to understand how the human immune system can develop longer-lasting protection against influenza.
FDA has approved Xospata (gilteritinib) to treat adult patients with relapsed or refractory acute myeloid leukemia with a certain genetic mutation.
A study conducted by MIT and the Massachusetts Eye and Ear has found glaucoma may be an autoimmune disorder.
FDA granted Sanofi Pasteur’s Adacel Tdap absorbed vaccine expanded indication to include repeat vaccinations for tetanus, diphtheria and pertussis.
A new survey shows more than 40 percent of Americans haven’t been vaccinated against the flu and aren’t planning to be due to misconceptions.