Industry News
Research, Science & Manufacturer Updates
The Centers for Medicare and Medicaid Services (CMS) has issued a final rule that updates the 2019 Medicare payment rates and wage index for hospices servicing Medicare beneficiaries.
The Centers for Medicare and Medicaid Services is launching the Medicare Advantage Qualifying Payment Arrangement Incentive demonstration.
The U.S. Food and Drug Administration (FDA) has approved Shire’s Cinryze (C1 esterase inhibitor [human]) to prevent angioedema attacks in children 6 years and older with hereditary angioedema.
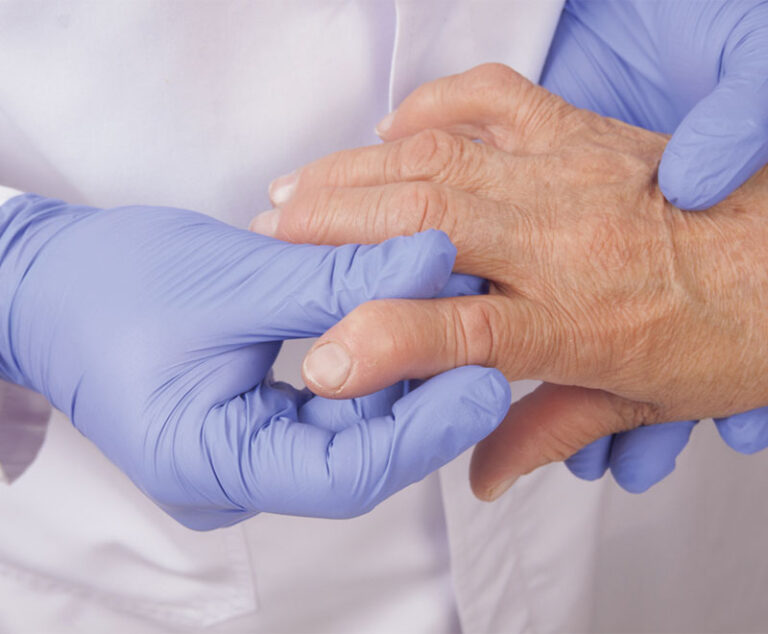
The U.S. Food and Drug Administration (FDA) has approved Incyte Corp.’s Olumiant (baricitinib) 2 mg once-daily oral medication to treat adults with moderately to severe active rheumatoid arthritis.
The U.S. Food and Drug Administration has approved the first prescription drug made from marijuana.
The U.S. Food and Drug Administration has approved new automated diagnostic tests for lupus and ANCA-associated vasculitis.
The U.S. Food and Drug Administration has approved Genentech’s Rituxan (rituximab) to treat moderate-tosevere phemphigus vulgaris.
The U.S. Food and Drug Administration (FDA) has approved a new, single-dose medication to treat people 12 years and older who have had the flu for no more than 48 hours.
FDA has approved the first generic versions of Mylan’s EpiPen and EpiPen Jr.
A new study shows newborns’ immune systems ramp up immediately after birth.
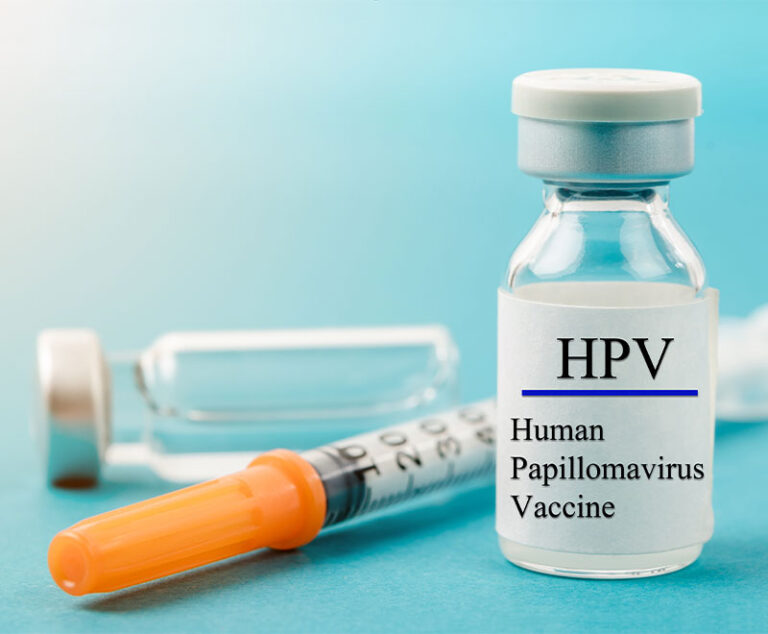
Merck’s Gardasil 9 human papillomavirus (HPV) vaccine has been approved by the U.S. Food and Drug Administration (FDA) for an expanded indication for men and women between the ages of 27 years and 45 years.
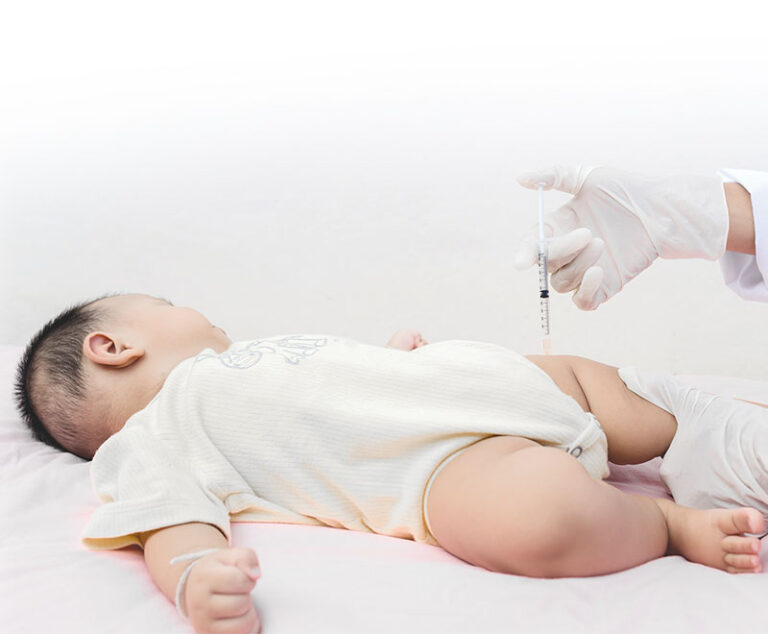
Researchers conducted a study that examined whether the childhood vaccine schedule was associated with an increased risk of infections not targeted by vaccines.