Industry News
Research, Science & Manufacturer Updates
Using technology that allows scientists to read the script of a person’s DNA, scientists have found a new autoimmune disease syndrome that combines severe lung disease and arthritis.
Baxalta has begun a Phase I, first-inhuman clinical trial of BAX 826, a recombinant factor VIII (rFVIII) treatment for hemophilia A that uses proprietary polysialic acid (PSA) technology to extend its circulating half-life.
Scientists at Yale have uncovered a molecular mechanism that causes variants in a specific immune response gene known as MIF (macrophage migration inhibitory factor).
A new tobacco-based seasonal influenza vaccine being developed by Mitsubishi Tanabe Pharma and currently in Phase III studies could potentially rival traditional chicken egg-based vaccines.
Analysis of 31 randomized controlled trials (RCTs) published between 1995 and 2015 found no evidence of increased thromboembolic event (TEE) risk among patients treated with intravenous immune globulin (IVIG) compared to control patients, according to a report by a team of investigators that included epidemiologists at the U.S. Food and Drug
Administration.
Scientists from Harvard, MIT and University College London have made a discovery about the genetics of cancer tumors that could offer a new way to deliver customized immunotherapy drugs to kill all types of cancer, including the most complex such as melanoma and lung cancer.
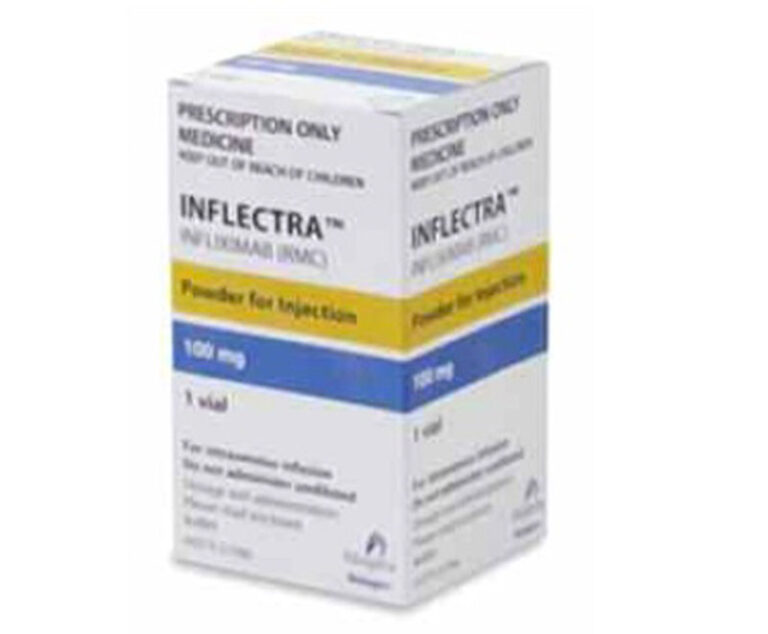
The U.S. Food and Drug Administration has approved Celltrion’s Inflectra (infliximab-dyyb), a biosimilar version of Johnson & Johnson’s Remicade drug used to treat autoimmune diseases.
Findings from a first in-human study for a new malaria vaccine candidate have shown a robust immune response while significantly delaying parasitemia.
The U.S. Food and Drug Administration has approved Opdivo (nivolumab) in combination with Yervoy (ipilimumab) for the treatment of patients with BRAFV600 wildtype and BRAFV600 mutation-positive unresectable or metastatic melanoma.
A new kind of gene therapy tested in animals could be safe and effective for human patients with hemophilia B.
Preliminary overall 2015-16 influenza vaccine effectiveness was 59 percent, according to the Centers for Disease Control and Prevention.