Industry News
Research, Science & Manufacturer Updates
University of Cincinnati researchers have discovered that a human cancer-causing gene, called DEK, can be detected in the plasma of head and neck cancer patients.
A new form of hybridized sound waves developed by Australian researchers may allow drugs and vaccines to be delivered to the body through a nebulizer in a fine mist inhaled into the lungs.
The U.S. Food and Drug Administration issued a new guidance recommending the deferral of individuals from donating blood if they have been to areas with active Zika virus transmission, potentially have been exposed to the virus or have had a confirmed Zika virus infection.
The U.S. Food and Drug Administration has approved Fluad (Seqirus), an influenza vaccine that contains the adjuvant MF59.
The U.S. Department of Health and Human Services (HHS) has announced additional funding under the Affordable Care Act (ACA) and other programs. Under the ACA, nearly $500 million is being awarded to health centers nationwide to provide primary care services to those who need them most.
Doctors who care for tens of millions of chronically ill Medicare patients are failing to take advantage of federal dollars intended to improve care and reduce hospital readmissions and overall costs, according to the Centers for Medicare and Medicaid Services (CMS).
A U.S.-Chinese research team pooled the results of four published studies that show among 445 people infected with either swine flu or H5N1 bird flu, those with a variant of a gene called IFITM3 were 24 percent more likely to have suffered a severe infection.
A study conducted between 2008 and 2014 at the Washington National Primate Research Center showed that vaccines did not cause any brain or behavioral changes in macaque monkeys.
Two studies presented at the International Conference on Emerging Infectious Diseases show that the influenza vaccine can protect for six months, last throughout the flu season and reduce hospitalization in children.
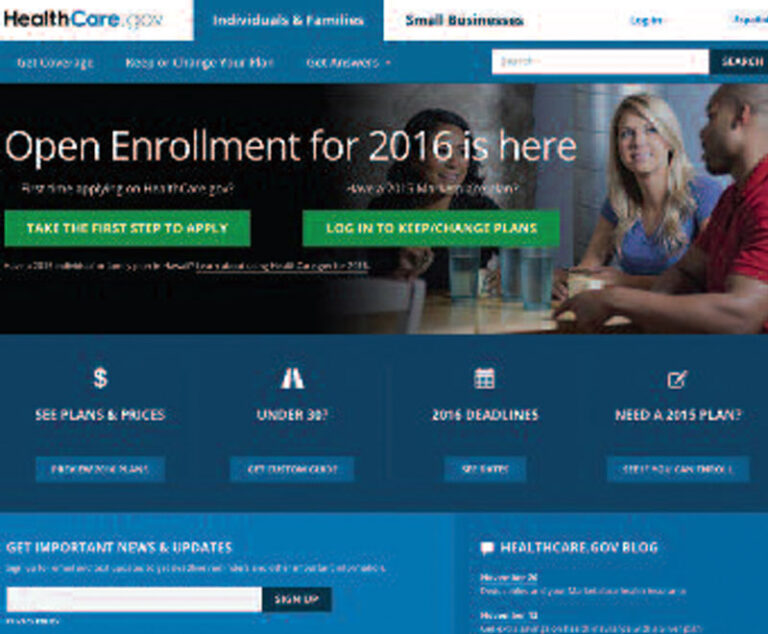
Healthcare.gov, the main entry point for people seeking insurance in 38 states served by the federal exchange, features three new window-shopping features.
The U.S. Food and Drug Administration ha approved Octapharma’s NUWIQ, antihemophilic factor (recombinant), an intravenous therapy for adults and children living with hemophilia A.
To make the U.S.healthcare system more accountable, the federal government continues to release Medicare claims and payment information.