Industry News
Research, Science & Manufacturer Updates
The Centers for Medicare and Medicaid Services issued final rules detailing how it will pay for services provided to Medicare beneficiaries in 2016.
In the Fall 2015 issue of BioSupply Trends Quarterly, the feature "The Perfect Storm for Patient-Centered Clinical Trials" included an incorrect statistic. Here is the correction.
Statin-triggered autoimmune myopathy can occur in rare instances where muscle-related symptoms fail to resolve following stoppage of the medication.
Hailed as a major advancement in the fight against skin cancer, the U.S. Food and Drug Administration (FDA) has approved a new immune-based therapy for treating metastatic melanoma.
President Obama signed a bill into law that will compensate patients for participating in clinical studies of rare diseases.
The U.S. Food and Drug Administration approved the first replacement therapy for hereditary factor X deficiency, coagulation factor X (Coagadex, Bio Products Laboratory), derived from human plasma.
The U.S. Food and DrugAdministration (FDA) approved BioProducts Laboratory’s Gammaplex(immune globulin intravenous [human]5% liquid) for pediatric patients 2 years of age and older who have primary immunodeficiency disease (PI).
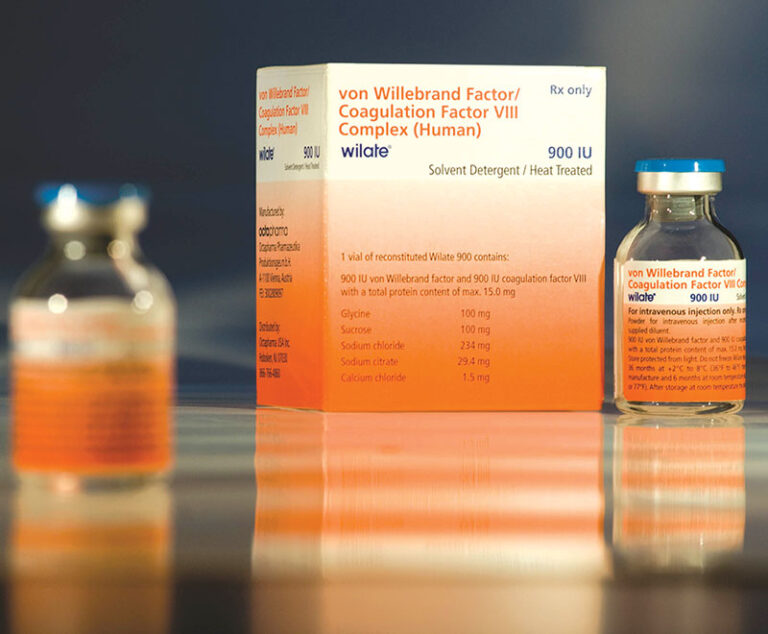
The U.S. Food and Drug Administration(FDA) has approved revised product labeling for Wilate (von Willebrand factor/coagulation factorVIII complex [human]) to include prevention of excessive bleeding during and after minor and major surgery in adult and pediatric von Willebrand disease patients.
A new report shows that stem cell transplants might soon offer multiple sclerosis (MS) patients an effective way to stave off relapses and improve their overall neurologic condition.
The U.S. Food and Drug Administration(FDA) has granted exclusivity to Flublok influenza vaccine for a period of 12 years.
Scientists at the University of South Australia and colleagues from Third Military Medical University in Chongqing, China, have found that the drug Edaravone alleviates Alzheimer’s disease pathologies at multiple levels and improves learning and memory functions in mice.
The Medicare Access and CHIP Reauthorization Act of 2015, which was enacted into law on April 16, will prohibit Medicare supplemental insurance(Medigap) policies from covering the Part B deductible for people who become eligible for Medicare on or afterJan. 1, 2020.