Industry News
Research, Science & Manufacturer Updates
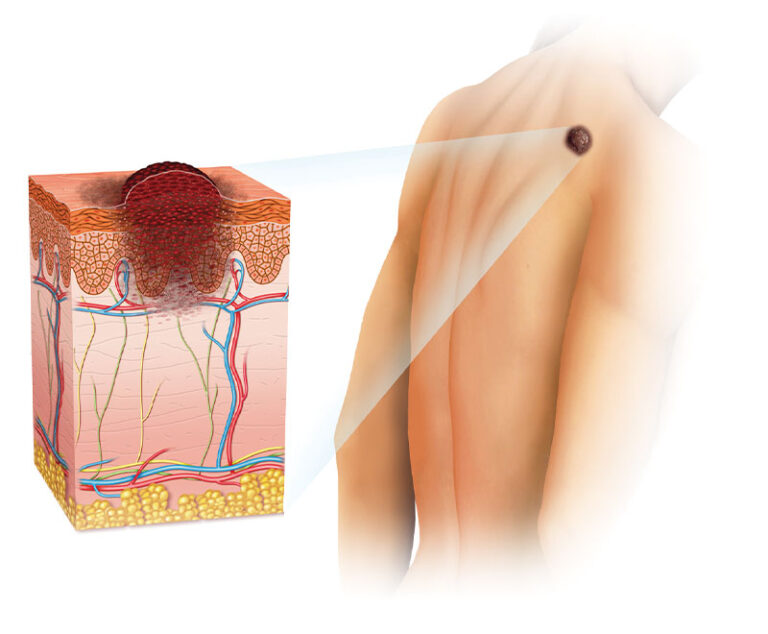
In two recent studies, researchers found that immune checkpoint inhibitors show promise in treating advanced melanoma.
A new study shows that people who receive a shingles vaccine but still contract shingles have a lower risk of developing post-herpatic neuralgia (PHN).
In a first-in-people clinical trial, personalized tailor-made melanoma vaccines given to three patients with advanced melanoma appeared to increase the number and diversity of cancer-fighting T cells responding to the tumors.
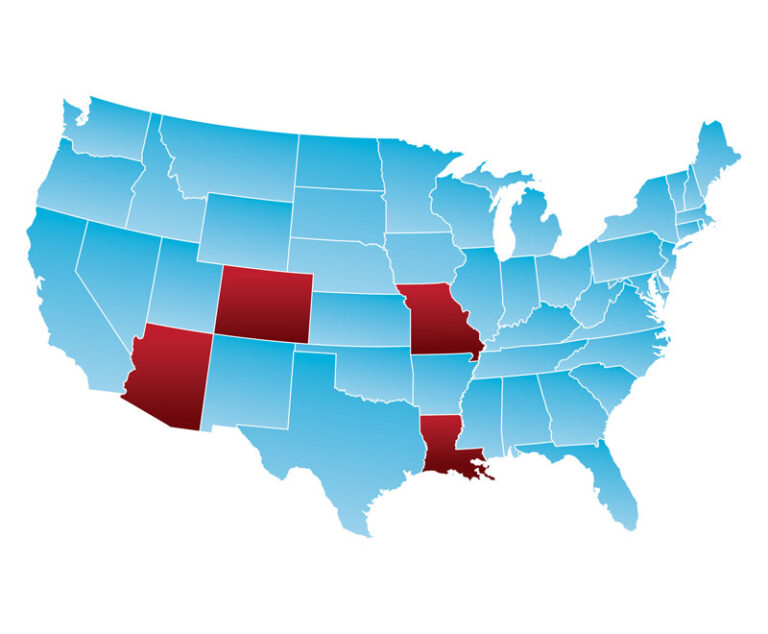
At its annual meeting in June, the American Medical Association (AMA) adopted a new policy to seek more stringent state immunization requirements to allow exemptions only for medical reasons.
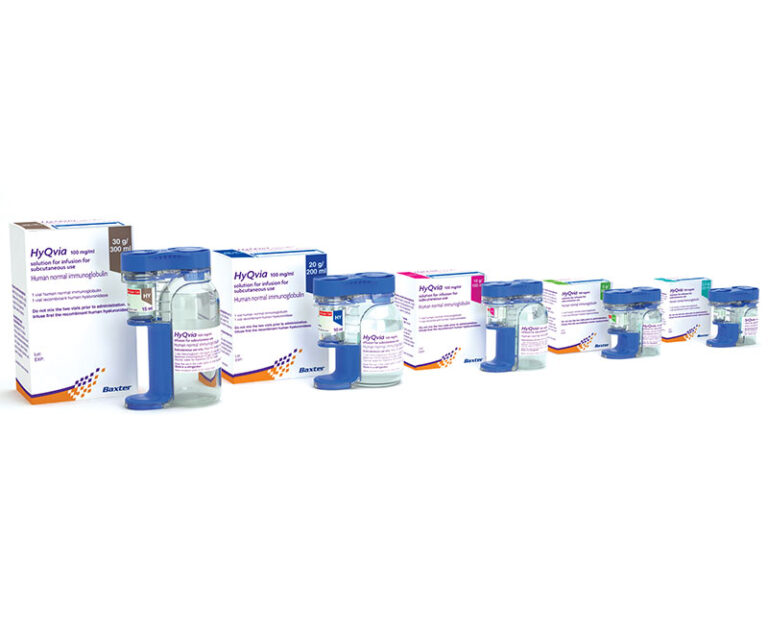
The Centers for Medicare and Medicaid Services (CMS) has expanded coverage to include in-home use of HYQVIA(immune globulin infusion 10 percent [human] with recombinant humanhyaluronidase) to treat primary immunodeficiency patients.
The U.S. FDA has issued a final rule requiring manufacturers to give six months’ notice if they plan to discontinue or interrupt production.
Pfizer (a licensee of Gliknik Inc.) has been granted orphan drug designation by the U.S. Food and Drug Administration (FDA) for its recombinant intravenous immune globulin (IVIG)-mimetic drug
GL-2045 to treat chronic inflammatory demyelinating polyneuropathy (CIDP).
Three recent studies have found that interventions increase the rates of human papillomavirus (HPV) vaccination among teens and young women.
CSL Behring’s Biologics License Application for the marketing authorization of its long-acting fusion protein linking recombinant coagulation factor IX with recombinant albumin, rIX-FP, has been accepted for review by the U.S.
Several states have introduced legislation that would let patients bypass the U.S. Food and Drug Administration’s (FDA) Expanded Access program in acquiring investigational therapies.
A final rule issued by the Centers for Medicare and Medicaid Services is intended to maintain the rigor of the Medicare Shared Savings program (created by the Affordable Care Act),while ensuring providers continue to participate.
A new simulation study that evaluated the relationship between Guillain-Barré syndrome (GBS) risk and influenza vaccine and illness suggests that the vaccine reduces the risk for GBS.