Industry News
Research, Science & Manufacturer Updates
Medicines Articles
The U.S. Food and Drug Administration has approved a recombinant ADAMTS13 protein product (Adzynma) as a prophylactic or on-demand enzyme replacement therapy for patients with congenital thrombotic thrombocytopenic purpura.
The U.S. Food and Drug Administration has approved IXCHIQ, Valneva’s single-dose, live-attenuated vaccine indicated for the prevention of disease caused by chikungunya virus in individuals 18 years of age and older who are at increased risk of exposure to CHIKV.
The U.S. Food and Drug Administration has approved Eli Lilly’s tirzepatide medication, which is branded as Mounjaro for diabetes, under a new brand for weight loss as well.
A new study shows efgartigimod significantly increased sustained platelet count responses compared with placebo in patients with chronic immune thrombocytopenia, including those who had received multiple previous immune thrombocytopenia therapies.
UCB Pharma’s investigational agent zilucoplan, a complement C5 inhibitor, to treat patients with myasthenia gravis has been approved by the U.S. Food and Drug Administration under the market name Zilbrysq.
Data suggests the bispecific BCMA-targeted antibody teclistamab may lead to grade 3 to 5 infections in as many as 44.8 percent of multiple myeloma patients, and hypogammaglobulinemia was noted in nearly 75 percent of patients.
The U.S. Food and Drug Administration (FDA) has approved nirsevimab to protect newborns from respiratory syncytial virus (RSV).
The U.S. Food and Drug Administration has approved Zurzuvae (zuranolone), the first oral medication indicated to treat postpartum depression (PPD) in adults.
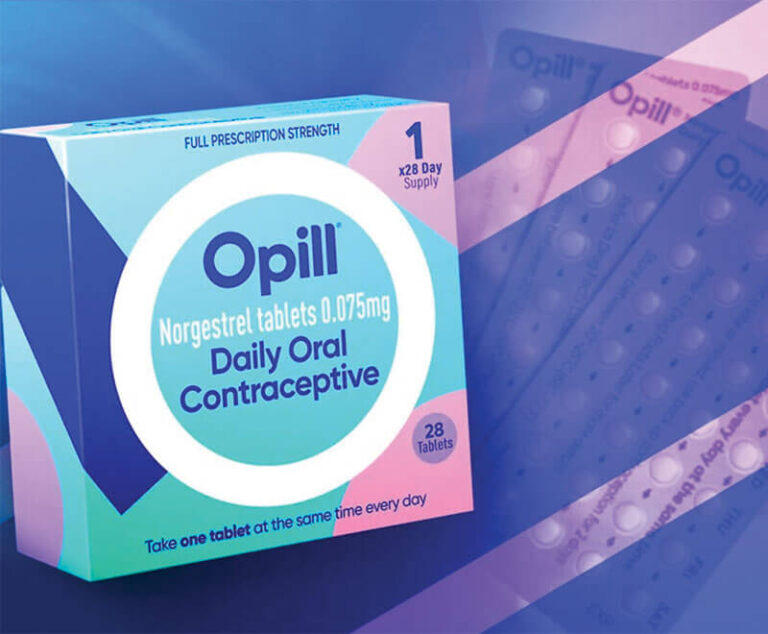
The U.S. Food and Drug Administration has approved the birth control pill Opill (norgestrel), manufactured by Perrigo, to be available over-the-counter — the first nonprescription birth control pill in the United States.
Pfizer's ABRYSVO, a respiratory syncytial virus (RSV) vaccine, has been approved by the U.S. Food and Drug Administration for the prevention of lower respiratory tract disease (LRTD) and severe LRTD caused by RSV in infants from birth up to 6 months of age by active immunization of pregnant women at 32 through 36 weeks gestational age.
The U.S. Food and Drug Administration (FDA) has converted Leqembi (lecanemab-irmb), indicated to treat adult patients with Alzheimer’s disease, to traditional approval following a determination that a confirmatory trial verified clinical benefit.
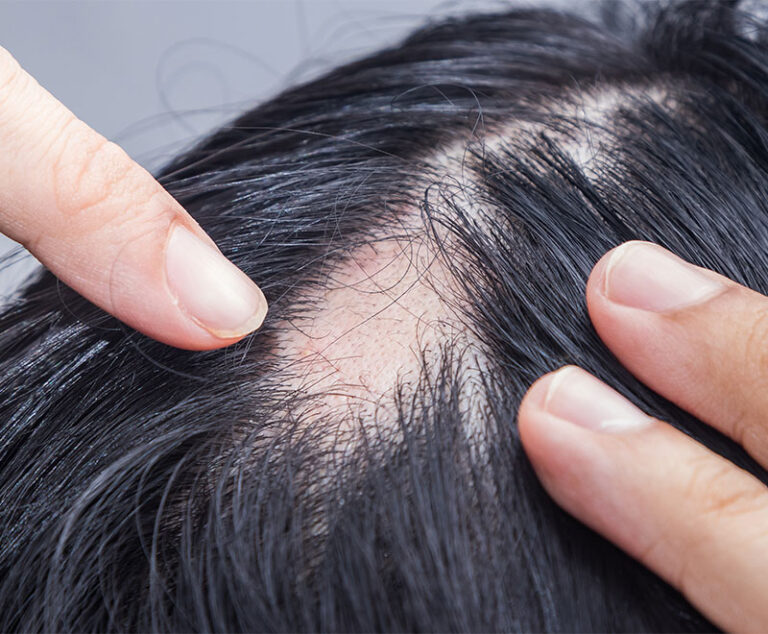
The U.S. Food and Drug Administration has approved LITFULO (ritlecitinib), a once-daily oral treatment, for individuals 12 years of age and older with severe alopecia areata.