Industry News
Research, Science & Manufacturer Updates
Medicines Articles
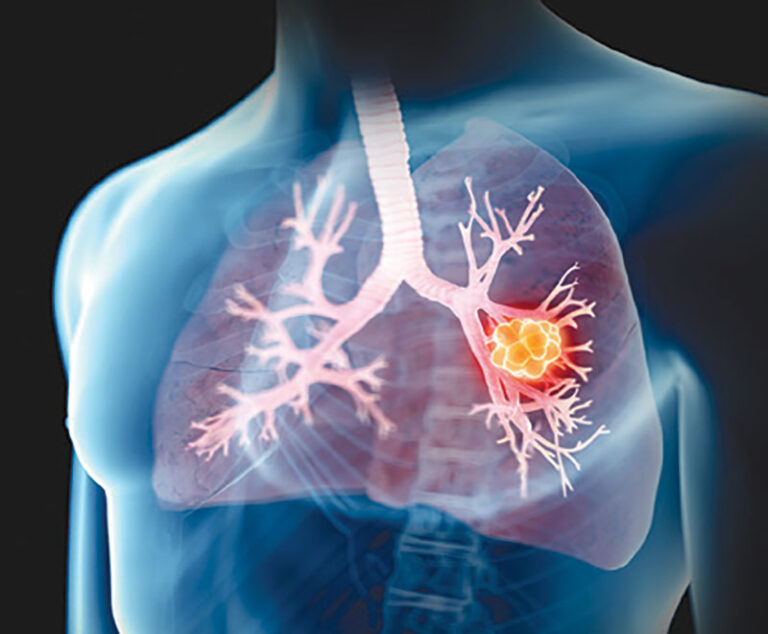
The U.S. Food and Drug Administration has approved Bristol Myers Squibb’s repotrectinib (Augtryo) for the treatment of adult patients with locally advanced or metastatic ROS1-positive non-small cell lung cancer.
The U.S. Food and Drug Administration has approved Tyenne (tocilizumab-aazg), a biosimilar referencing tocilizumab (Actemra; Genentech), to treat multiple autoimmune diseases, including rheumatoid arthritis and juvenile idiopathic arthritis.
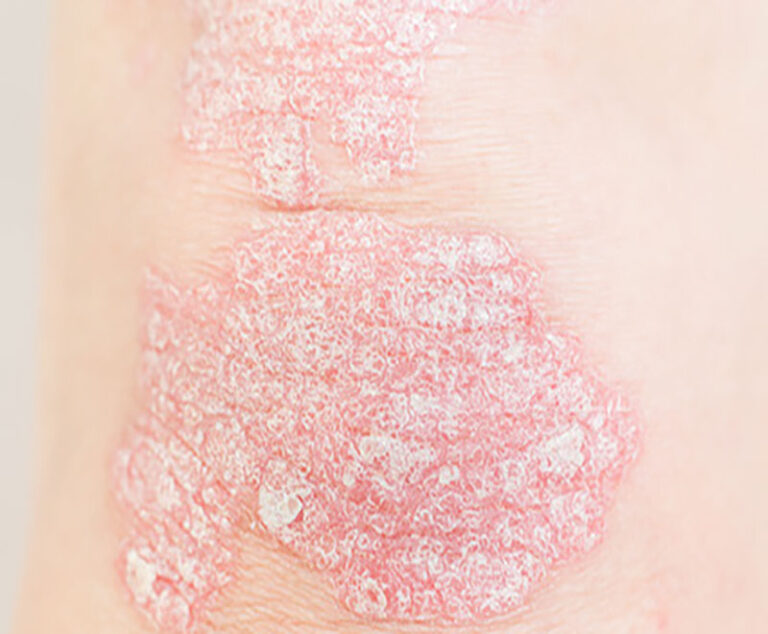
The U.S. Food and Drug Admini-stration has approved Selarsdi (ustekinumab-aekn) injection for subcutaneous use, as a biosimilar to Stelara, for the treatment of moderate to severe plaque psoriasis and for active psoriatic arthritis in adults and pediatric patients 6 years and older.
The U.S. Food and Drug Administration (FDA) has granted orphan drug exclusivity for Octapharma’s wilate, von Willebrand factor/coagulation factor VIII complex (human) lyophilized powder for solution for intravenous injection, for routine prophylaxis to reduce the frequency of bleeding episodes in adults and children 6 years of age and older with von Willebrand disease (VWD).
Biotest has received approval from the U.S. Food and Drug Administration for Yimmug to treat primary immunodeficiencies in patients 2 years and older.
Investigators at the Bern University Hospital conducted a single-center randomized clinical trial to quantify and compare the PVE properties of iso-oncotic 5% albumin, hyper-oncotic 20% albumin and Ringer’s lactate (RL) in patients undergoing radical cystectomy.
The Phase IIIb study of emicizumab shows promise for reducing risk of spontaneous and traumatic bleeds in infants with hemophilia A.
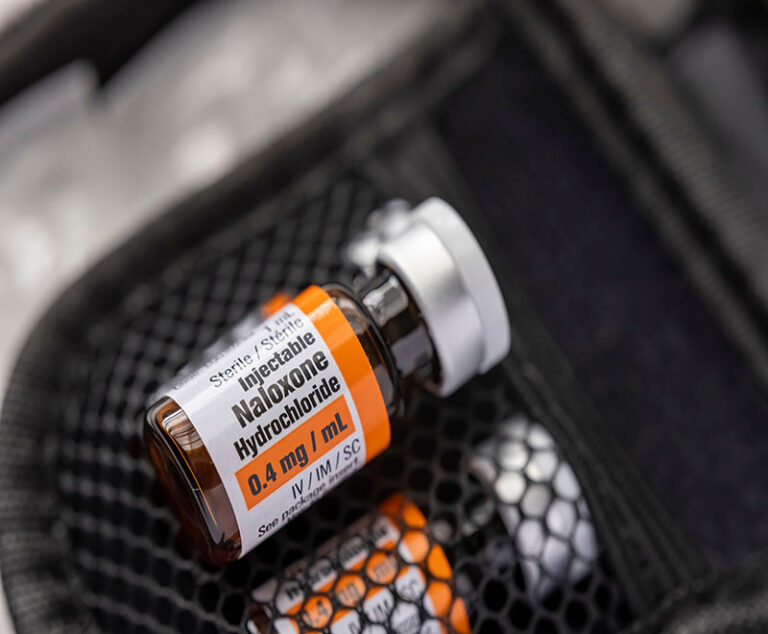
FDA approved and implemented updated warning labels for all opioid prescriptions.
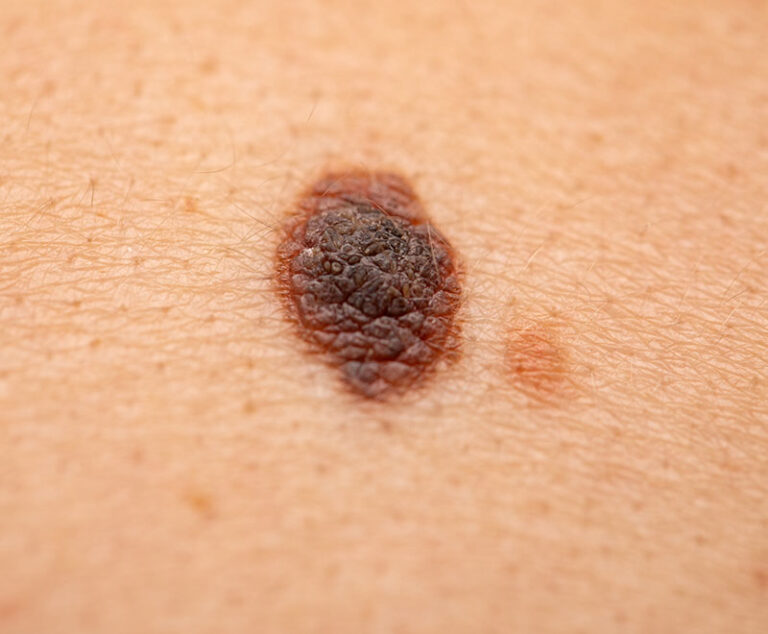
FDA approved Amtagvi, the first cellular therapy indicated for the treatment of adult patients with unresectable melanoma or metastatic melanoma that previously has been treated with other therapies.
FDA has approved Alvotech’s Simlandi (adalimumab-ryvk) as the third interchangeable Humira biosimilar.
The U.S. Department of Health and Human Services and the General Services Administration have announced new guidance recommending that all federal facilities across the nation include overdose reversal medications in their safety stations on site.
The U.S. Food and Drug Administration has authorized the use of Eli Lilly & Co.’s Zepbound for adults with obesity and moderate to severe obstructive sleep apnea, a common condition where a person struggles to breathe properly during sleep.