Industry News
Research, Science & Manufacturer Updates
Eight consecutive patients with acute-onset purpura fulminans (PF) secondary to systemic bacterial infection survived following treatment with a licensed human plasma-derived protein C concentrate (Ceprotin, Baxter Healthcare) in conjunction with standard sepsis therapy.
An analysis of 67 research studies published in the July 1 edition of Pediatrics has found that serious complications related to vaccines are very rare, and there is no evidence that immunizations cause autism.
The Department of Health andHuman Services (HHS) will allow certain hospitals to receive discounts on orphan drugs when they are used for non-orphan conditions, despite a ruling by the U.S. District Court for the District of Columbia that HHS did not have the authority to do so.
bioCSL has announced the donation of more than 700,000 doses of its influenza vaccine to the Partnership for Influenza Vaccine Introduction.
A substantial decrease in Medicare spending as a result of improvements made by the Affordable Care Act (ACA)will result in the Medicare Trust Fund lasting until 2030.
FDA has approved Iroko Pharmaceuticals’ Tivorbex (indomethacin), a low-dose painkiller for adult patients.
A federal appeals court has struck down subsidies provided under the Affordable Care Act (ACA) for federally run insurance exchanges.
The Society for Clinical Research Sites (SCRS) and TransCelerate BioPharma Inc. have launched the Site Advocacy Group (SAG), an initiative that enables clinical investigators and site professionals to interact directly with senior industry leaders in the exchange of perspectives and experiences on innovative ideas, processes, tools and technologies.
The Advisory Committee on Immunization Practices voted 15 to 0 to recommend a preference for the inhaled live attenuated influenza vaccine, FluMist Quadrivalent, for healthy children ages 2 years through 8 years.
The U.S. Food and Drug Administration has approved HYQVIA (Immune Globulin Infusion 10% [Human] with Recombinant Human Hyaluronidase), Baxter’s subcutaneous treatment for adult patients with primary immunodeficiency.
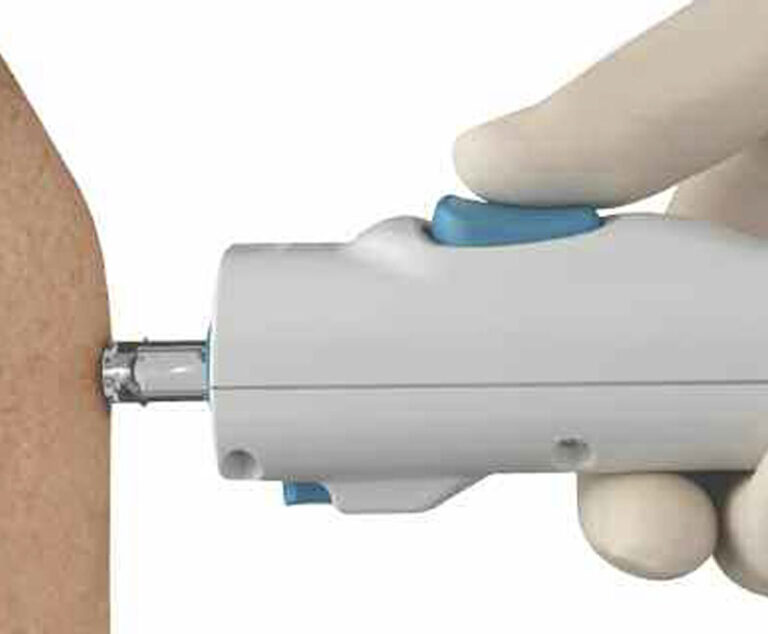
The U.S. Food and Drug Administration (FDA) has approved PharmaJet Inc.’s Stratis 0.5mL Needle-Free Jet Injector for delivery of bioCSL Inc.’s AFLURIA influenza vaccine for individuals ages 18 to 64 years.
Under a proposed rule by the Centers for Medicare and Medicaid Services(CMS), healthcare providers will have an extra year to use 2011 edition software in their electronic health record systems under the federal incentive program for health IT.