Industry News
Research, Science & Manufacturer Updates
U.S. Health and Human Services Secretary Kathleen Sebelius recognized more than 900 Champions for Coverage organizations nationwide in September.
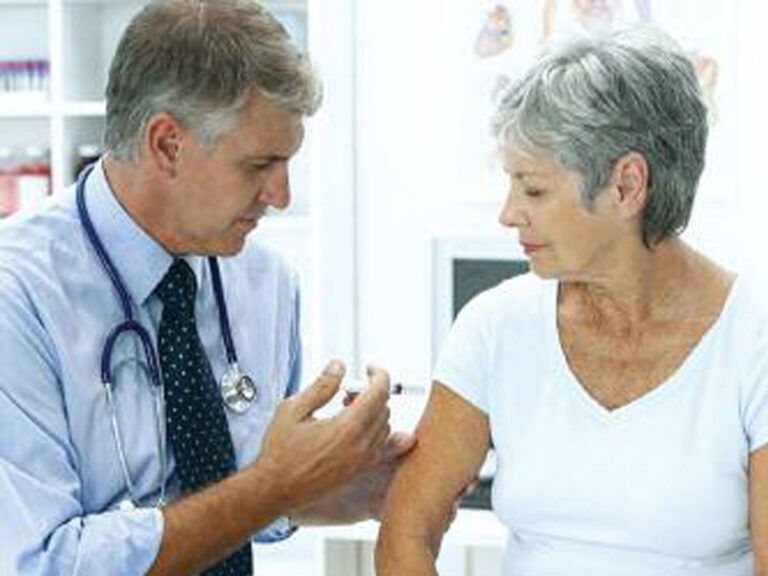
Results from a large clinical trial of Sanofi Pasteur’s Fluzone High-Dose showed that the influenza vaccine was 24.2 percent more effective against lab-confirmed flu than the traditional vaccine dose in adults 65 and older.
Novavax Inc. announced positive preclinical data for its virus-like particle (VLP) vaccine candidate against A(H7N9) influenza.
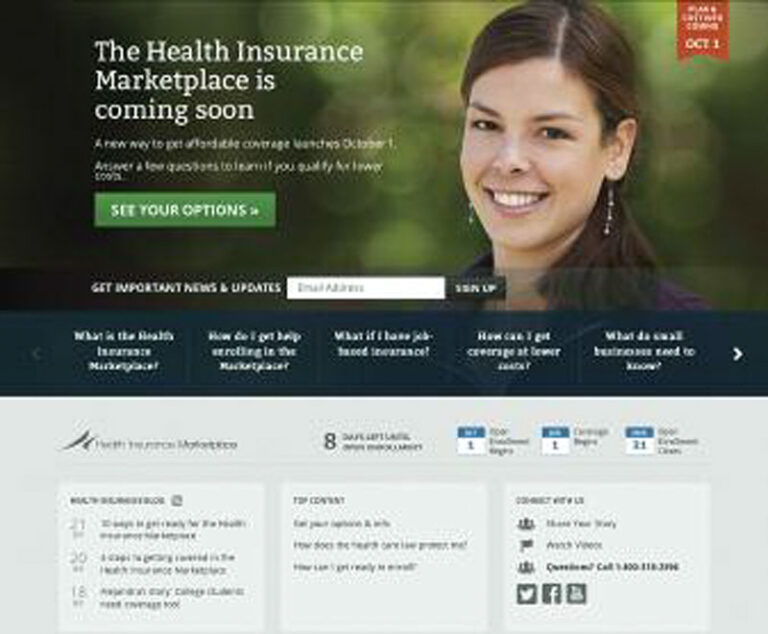
In June 2013, the Obama administration launched the consumer-focused HealthCare.gov website and the 24-hour call center in an effort to help Americans successfully navigate the Health Insurance Marketplace.
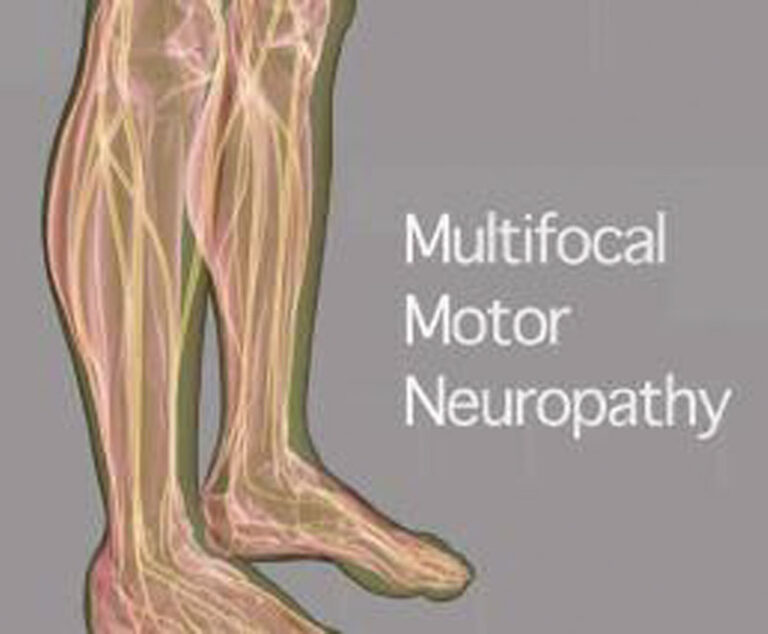
The Neuropathy Action Foundation and the GBS/CIDP Foundation International have launched a joint nationwide campaign to raise awareness of multifocal motor neuropathy.
Novovax Inc.’s quadrivalent seasonal influenza virus-like particle (VLP) vaccine demonstrated the company’s PhaseII clinical trial’s primary endpoint of safety and immunogenicity of three ascending dose levels.
In a prospective, multicenter, single-arm, open-label PhaseIII study, 61 percent of patients with chronic inflammatory demyelinating polyneuropathy (CIDP) responded to induction and maintenance therapy with a 10% liquid human intravenous immunoglobulin (IVIG) (Privigen, CSL Behring).
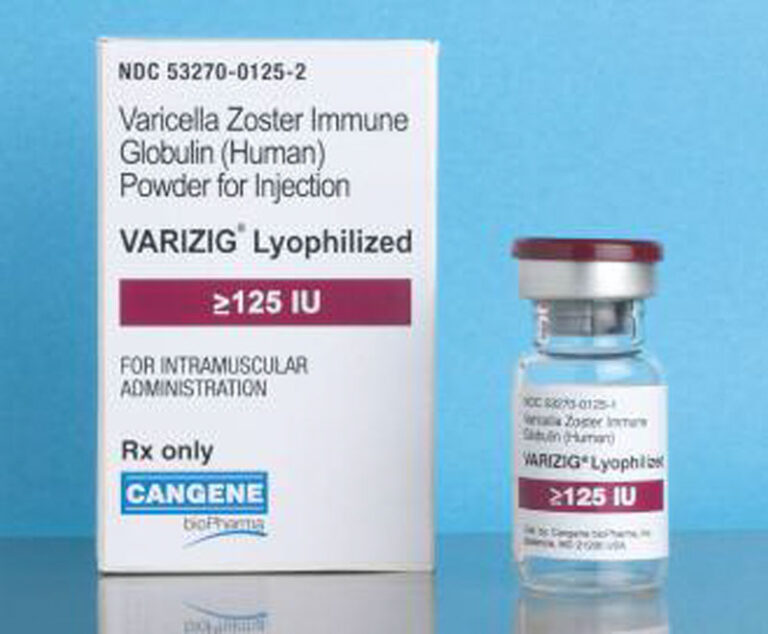
The Centers for Disease Control and Prevention has updated its recommendations for the use of VariZIG.
A recent study conducted to evaluate barriers to pregnant women’s uptake of the influenza vaccine found that the decline in vaccination among this population was due to lower levels of knowledge and unfavorable attitudes regarding the vaccine’s safety and efficacy.
The Department of Health and Human Services and Young Invincibles launched a video contest to inform young people about health insurance coverage under the Affordable Care Act.
A study by researchers at RTI International and the U.S. Centers for Disease Control and Prevention showed that two-dimensional barcodes on vaccine product labels would enhance the safety of the U.S. immunization system and save more than $300 million by 2023.
The U.S. Food and Drug Administration has approved a 10 g (50mL) vial size for Hizentra, immune globulin subcutaneous (human).