Industry News
Research, Science & Manufacturer Updates
Disease Indications Articles
The U.S. Food and Drug Administration (FDA) has approved nirsevimab to protect newborns from respiratory syncytial virus (RSV).
A study led by scientists at Oregon Health & Science University has found that a type of monoclonal antibody already tested in certain forms of cancer may be a promising treatment in stopping the progression of amyotrophic lateral sclerosis (ALS).
The U.S. Department of Health and Human services has awarded more than $147 million to 49 recipients to advance the Ending the HIV Epidemic in the U.S. initiative, which is part of the ongoing efforts to reduce the number of new infections in the United States by at least 90 percent by 2030.
An expanded indication for ERVECO for the prevention of disease caused by Zaire ebolavirus in individuals 12 months of age and older has been approved by the U.S. Food and Drug Administration.
A recent study has found chronic immune-related side effects are common in patients with skin cancer who are treated with postsurgical Opdivo (nivolumab) or Keytruda (pembrolizumab), although for some individuals, these toxicities resolve by the 18-month mark.
Recent case series findings, as well as previous studies, show that children with multisystem inflammatory syndrome (MIS-C) who present with signs of active neurological symptoms may show improvement with intravenous immune globulin (IVIG) and corticosteroids.
A new study conducted at the University of Texas Health Science Center at Houston found that people who received shingles and pneumonia vaccines — along with tetanus and diphtheria — had as much as a 30 percent reduced risk of developing Alzheimer's the most common type of dementia.
Pfizer's ABRYSVO, a respiratory syncytial virus (RSV) vaccine, has been approved by the U.S. Food and Drug Administration for the prevention of lower respiratory tract disease (LRTD) and severe LRTD caused by RSV in infants from birth up to 6 months of age by active immunization of pregnant women at 32 through 36 weeks gestational age.
A new study shows that a personalized messenger RNA (mRNA) cancer vaccine plus the checkpoint inhibitor Keytruda (pembrolizumab) reduced the risk of recurrence or death in people with high-risk advanced melanoma.
A large clinical trial of Eli Lilly's experimental Alzheimer's medication, donanemab found the drug slowed declines in patients' ability to think clearly and perform daily tasks by more than a third.
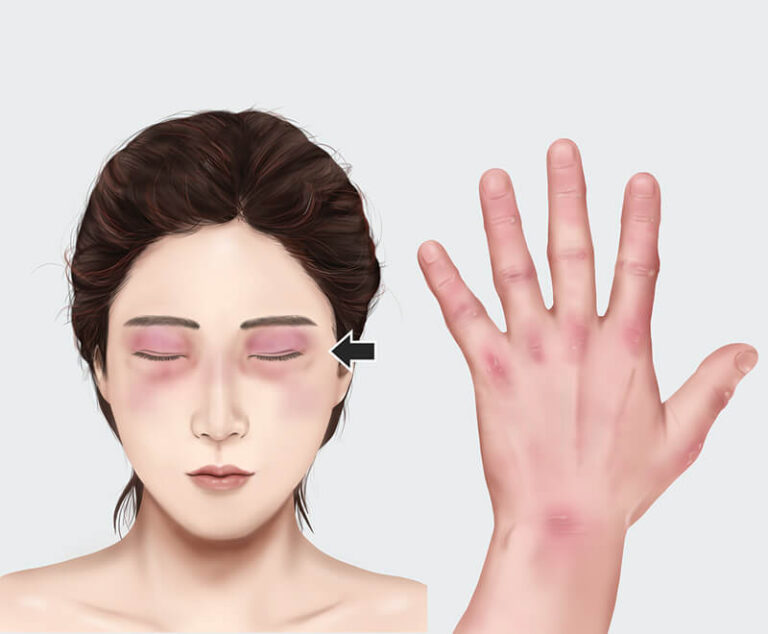
A study evaluating intravenous immune globulin for the treatment of dermatomyositis has found it significantly improved patient outcomes.
Regen BioPharma has filed a provisional patent application covering utilization of dendritic cell technologies to augment efficacy of its patented mRNA cancer immunotherapeutic vaccine.