Industry News
Research, Science & Manufacturer Updates
As the struggle over the details of the Affordable Care Act persists, changes continue to be made.
A multinational European research team has reported findings from a randomized controlled trial that compared the efficacy of prophylaxis with episodic (on-demand) therapy in children with severe hemophilia over a 10-year time period.
Updates on recent FDA approvals, appointments, acquisitions and alliances in the pharmaceutical industry.
A new law is being proposed that would allow Medicare patients who have a chronic condition to review all their medications in one-on-one sessions with pharmacists, helping them to stick to their drug regimen.
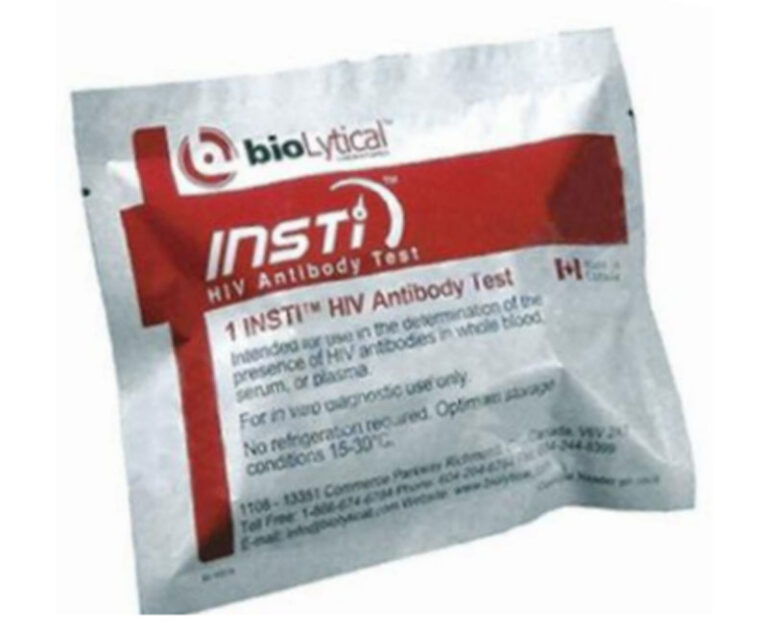
The U.S. Food and Drug Administration has approved a single-use rapid test that detects antibodies to HIV-1 in as little as 60 seconds.
Several recent studies have proven gene therapy is successful in treating immune diseases.
The U.S.Food and Drug Administration has approved a supplemental Biologics License Application to extend the shelf life of Hizentra.
Twenty-one Republican governors signed a letter sent in February to U.S.Department of Health and Human Services Secretary Kathleen Sebelius asking for more flexibility for states as they put together their own health exchanges.
According to the Centers for Disease Control and Prevention, vaccination rates for adults are lower than they should be.
A new study suggests that individuals with the autoimmune skin disease bullous pemphigoid appear more likely to have a diagnosis of neurologic disease.
A new study suggests prenatal use of influenza vaccine may protect young infants against the flu.
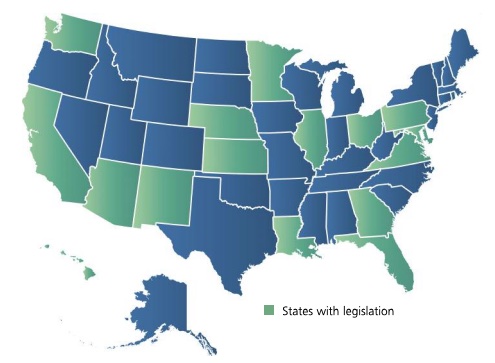
The introduction of legislation to ensure access by patients to high-cost therapies continues to increase throughout the country.