New Test Can Accurately Predict 90 Percent of Alzheimer’s Cases

A new study has found a combined blood test for cognitive decline has a 90 percent accuracy rate in determining whether memory loss is due to Alzheimer’s disease.
Deuruxolitinib Approved by FDA to Treat Severe Alopecia
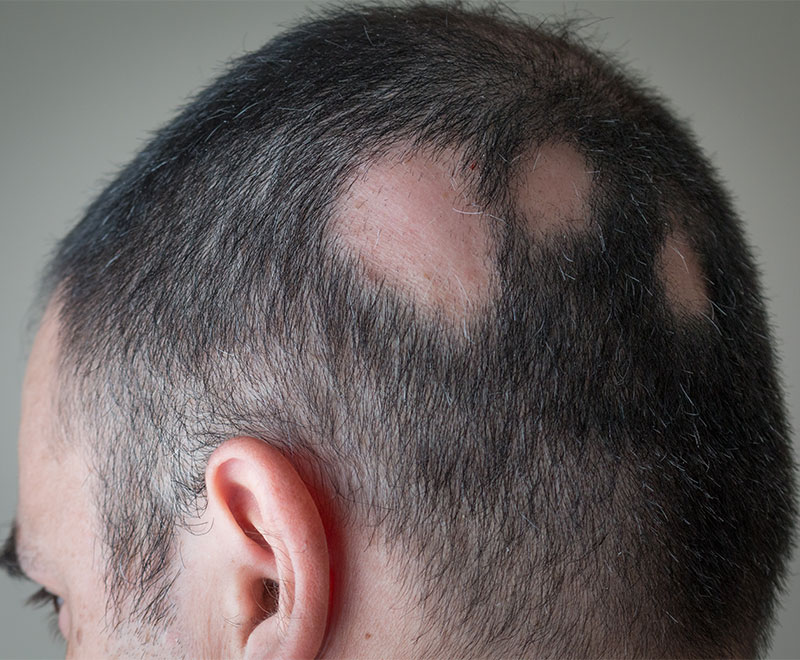
Sun Pharma’s LEQSELVI (deuruxolitinib) has been approved by the U.S. Food and Drug Administration to treat severe alopecia areata in adults.
FDA Approves Epysqli, Second Biosimilar to Soliris

Samsung Bioepis’ biologics license application for Epysqli (eculizumab-aagh) as a biosimilar to Soliris (eculizumab) has been approved by the U.S. Food and Drug Administration (FDA) to treat patients with paroxysmal nocturnal hemoglobinuria (PNH) to reduce hemolysis and atypical hemolytic uremic syndrome (aHUS) to inhibit complement-mediated thrombotic microangiopathy.
Universal Flu Vaccine May Be Available in Five Years

New research reveals a promising approach to a universal influenza vaccine vaccine that confers lifetime immunity against an evolving virus.
Drug to Treat Acute Pancreatitis Granted Fast Track Designation by FDA
Panafina Inc.’s investigational new drug, RABI-767, has been granted fast track designation by the U.S. Food and Drug Administration (FDA) to treat patients with acute pancreatitis predicted to progress to severe disease.
Study Shows Vaccination Decision in IEI Patients Must Be Individualized

Inborn errors of immunity (IEI) increase morbidity and mortality risks, particularly from respiratory tract infections. Hence, vaccination becomes pivotal for IEI patients.
Immune Globulin and Prophylactic Antibiotics Provide Similar Efficacy in Treating Hypogammaglobulinemia Secondary to Hematological Malignancy
Immune globulin replacement and prophylactic antibiotics are commonly used to prevent infections in patients with secondary hypogammaglobulinemia due to hematological malignancies but have never been directly compared.
New Study to Examine COVID-19 Vaccines in People with Weakened Immune Systems
Researchers at the University of Wisconsin (UW) School of Medicine and Public Health are exploring the ideal vaccine booster strategy for immunosuppressed patients to protect those at higher risk of severe illness and complications from COVID-19 infection.
New Vaccine Could Lower ‘Bad’ Cholesterol by as Much as 30 Percent
A new vaccine currently in development can effectively and affordably lower levels of “bad’ cholesterol in the body, a health problem that affects almost two in five adults in the U.S.
CSL Behring Adds 4 and 5 Gram Vials of ZEMAIRA

CSL Behring’s ZEMAIRA (alpha1- proteinase inhibitor [human]) is now available in 4 gram and 5 gram vials.