Industry News
Research, Science & Manufacturer Updates
FDA Updates Articles
CSL Behring’s Biologics License Application for the marketing authorization of its long-acting fusion protein linking recombinant coagulation factor IX with recombinant albumin, rIX-FP, has been accepted for review by the U.S.
The U.S. Food and Drug Administration(FDA) has finalized three guidance documents outlining its expectations for biosimilars.
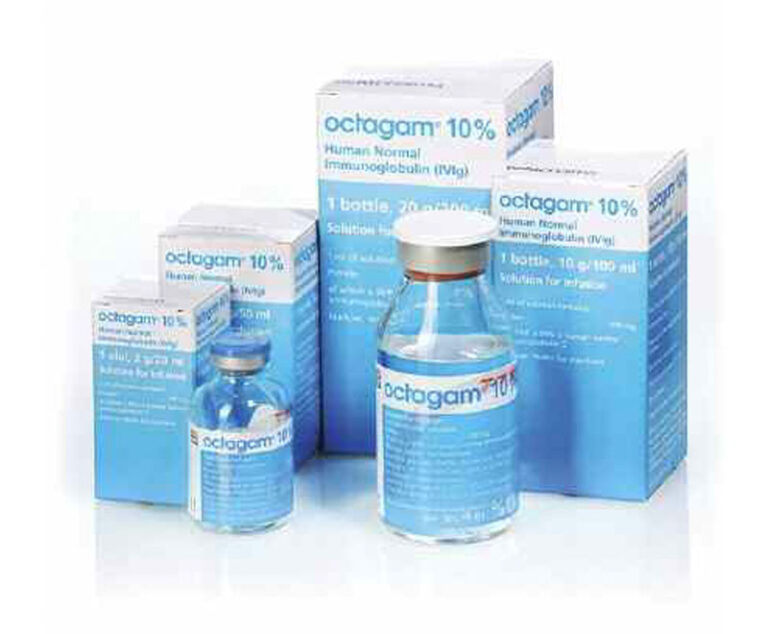
The U.S. Food and Drug Administration(FDA) has approved Octapharma’s manufacturing facility in Vienna, Austria, for the production of Octagam 10% (immune globulin intravenous [human] 10% [100mg/mL] liquid preparation).
FDA has approved Iroko Pharmaceuticals’ Tivorbex (indomethacin), a low-dose painkiller for adult patients.
The U.S. Food and DrugAdministration has approved obinutuzumab (Gazyva, Genentech)for the treatment of patients with previously untreated chronic lymphocyticleukemia.
The U.S. Food and Drug Administration has approved two new quadrivalent influenza vaccines (IIV4s).
The U.S. Food and Drug Administration (FDA) has determined that hydroxyethyl starch (HES) solutions should not be used in critically ill adult patients, including patients with sepsis and those admitted to the intensive care unit (ICU).
The U.S. Food and Drug Administration(FDA) has approved an expanded indication for CSL Behring’s Corifact, factor XIII (FXIII) concentrate (human) to include the perioperative management of surgical bleeding in adult and pediatric patients with congenital FXIII deficiency.
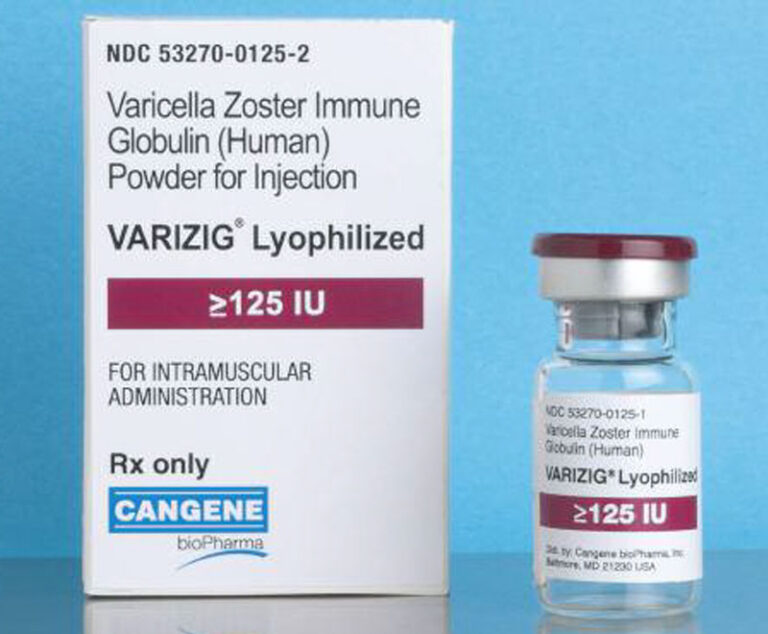
VARIZIG, a hyperimmune globulin indicated for post-exposure prophylaxis of varicella zoster virus (VZV) in high-risk patients, has been approved by the U.S.Food and Drug Administration.
The U.S. Food and Drug Administration has approved a new children’s vaccine that targets two common causes of bacterial meningitis.
Octapharma USA’s Octaplas, a solvent/detergent-treated pooled human plasma, has been approved by the U.S. Food and Drug Administration.
The U.S. Food and Drug Administration has approved Fluarix Quadrivalent to immunize children ages 3 and older and adults against flu virus subtypes A and B.