Industry News
Research, Science & Manufacturer Updates
FDA Updates Articles
The U.S. Food and Drug Administration has approved Baxalta’s Adynovate for use in hemophilia A patients aged 12 years and older.
The U.S. Food and Drug Administration issued a new guidance recommending the deferral of individuals from donating blood if they have been to areas with active Zika virus transmission, potentially have been exposed to the virus or have had a confirmed Zika virus infection.
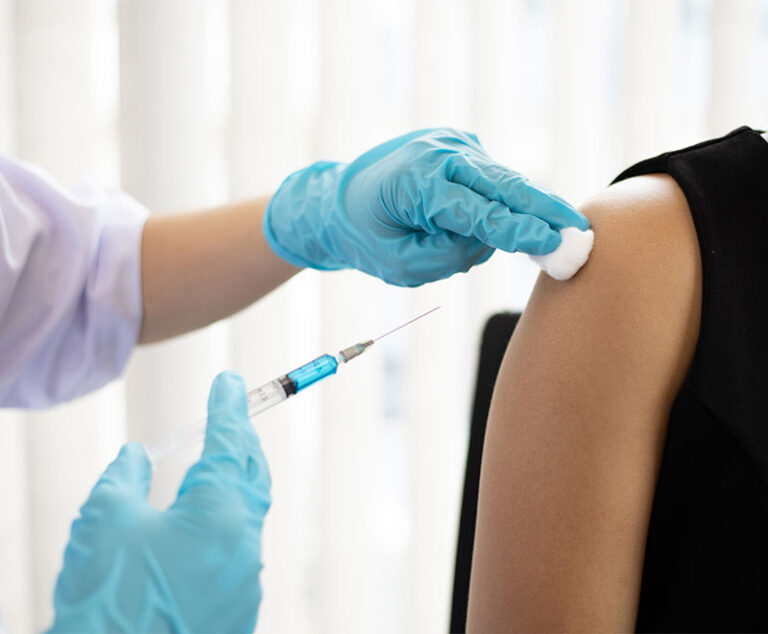
The U.S. Food and Drug Administration has approved Fluad (Seqirus), an influenza vaccine that contains the adjuvant MF59.
The U.S. Food and Drug Administration ha approved Octapharma’s NUWIQ, antihemophilic factor (recombinant), an intravenous therapy for adults and children living with hemophilia A.
The U.S. Food and Drug Administration has approved Belbuca (buprenorphine) buccal film for patients with severe chronic pain.
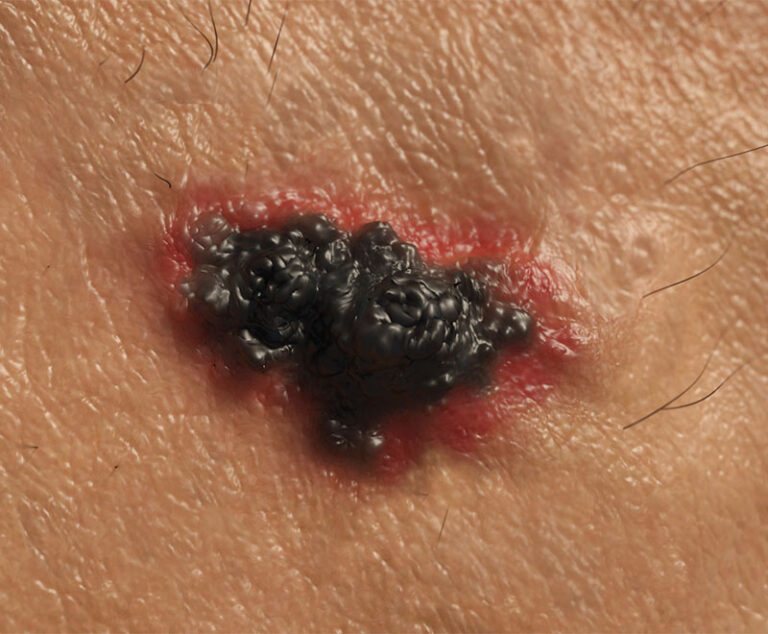
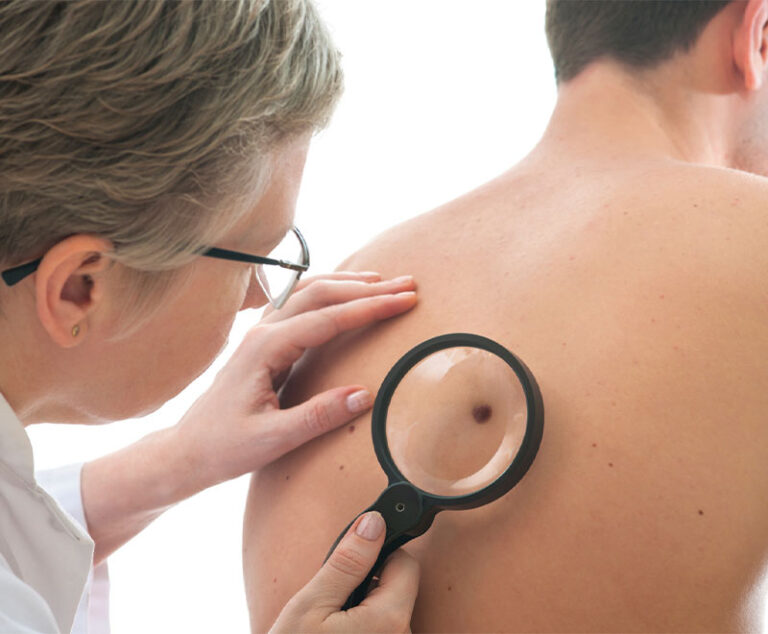
The U.S. Food and Drug Administration (FDA) approved Bristol-Myers Squibb’s Opdivo (nivolumab) in combination with Yervoy (ipilimumab) for the treatment of advanced melanoma.
Hailed as a major advancement in the fight against skin cancer, the U.S. Food and Drug Administration (FDA) has approved a new immune-based therapy for treating metastatic melanoma.
The U.S. Food and Drug Administration approved the first replacement therapy for hereditary factor X deficiency, coagulation factor X (Coagadex, Bio Products Laboratory), derived from human plasma.
The U.S. Food and DrugAdministration (FDA) approved BioProducts Laboratory’s Gammaplex(immune globulin intravenous [human]5% liquid) for pediatric patients 2 years of age and older who have primary immunodeficiency disease (PI).
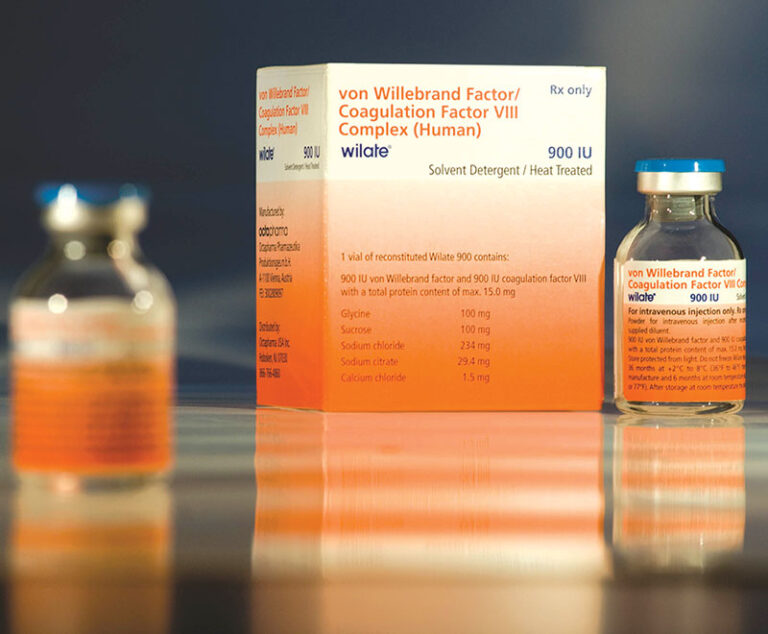
The U.S. Food and Drug Administration(FDA) has approved revised product labeling for Wilate (von Willebrand factor/coagulation factorVIII complex [human]) to include prevention of excessive bleeding during and after minor and major surgery in adult and pediatric von Willebrand disease patients.
The U.S. Food and Drug Administration(FDA) has granted exclusivity to Flublok influenza vaccine for a period of 12 years.
Pfizer (a licensee of Gliknik Inc.) has been granted orphan drug designation by the U.S. Food and Drug Administration (FDA) for its recombinant intravenous immune globulin (IVIG)-mimetic drug
GL-2045 to treat chronic inflammatory demyelinating polyneuropathy (CIDP).