Industry News
Research, Science & Manufacturer Updates
Medicines Articles
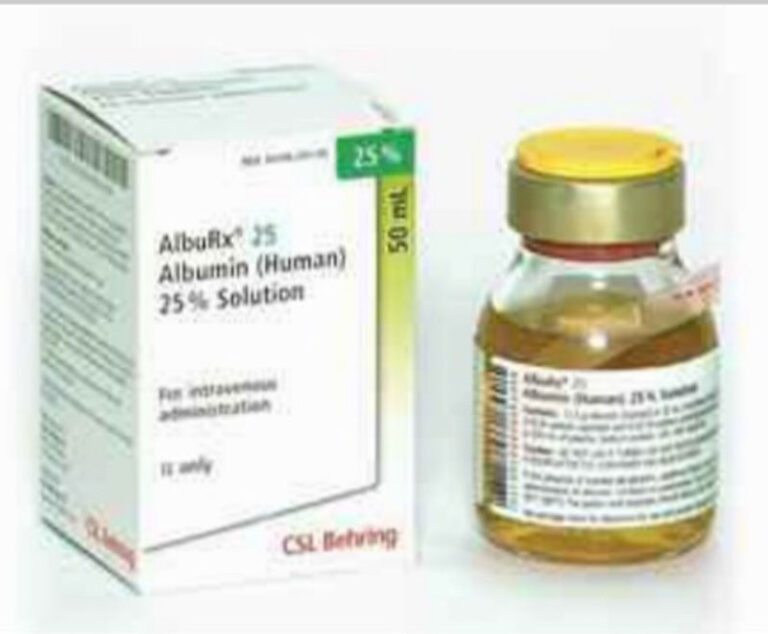
CSL Behring noted the potential for fading print with more effect on the expiration dating on the patient tear-off portion of the vial label.
Researchers have found antibiotics can be counterproductive and weaken the immune system’s ability to fight off bacteria.
A nationwide epidemiological study showed cancer patients who continuously used disulfiram (Antabuse), a drug prescribed to alcoholics to prevent them from drinking, have a lower risk of death from cancer compared to those who stopped using the drug once diagnosed.
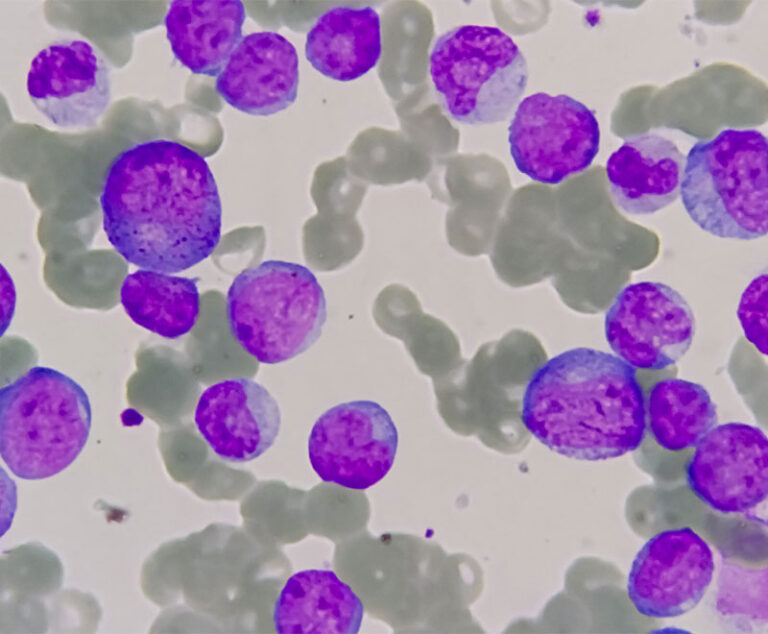
The U.S. Food and Drug Administration has approved Agios Pharmaceuticals’ Idhifa (enasidenib) to treat acute myelogenous leukemia.
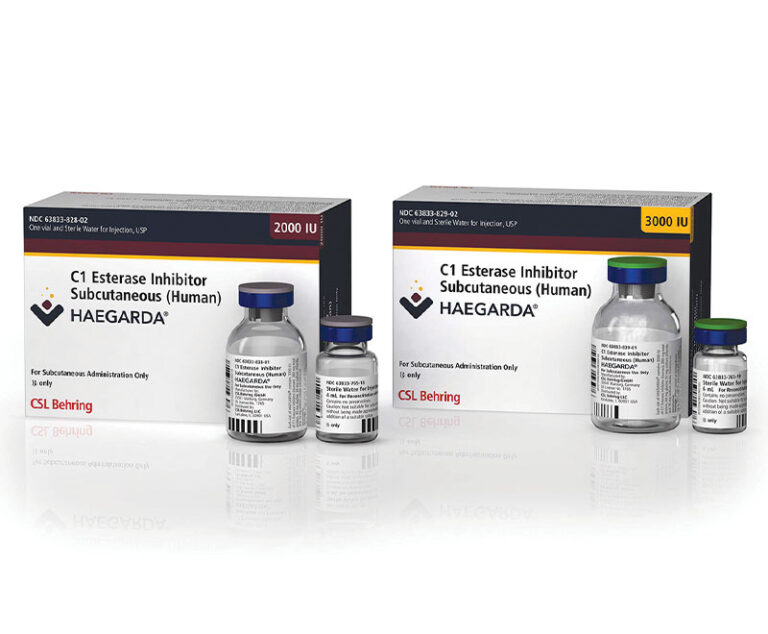
The U.S. Food and Drug Administration has approved CSL Behring’s Haegarda (C1 esterase inhibitor subcutaneous [human]), the first and only subcutaneous therapy indicated for routine prophylaxis to prevent hereditary angioedema attacks in adolescent and adult patients.
The U.S. Food and Drug Administration (FDA) has approved Privigen (immune globulin intravenous [human] 10% liquid) to treat adults with chronic inflammatory demyelinating polyneuropathy (CIDP) to improve neuromuscular disability.
The U.S. Food and Drug Administration has approved AbbieVie Inc.’s Mavyret (glecaprevir and pibrentasvir) to treat adults with certain types of hepatitis C.
New rules issued by the U.S. Department of Health and Human Services give employers more leeway to withhold birth control coverage on religious grounds.
The U.S. Food and Drug Administration has approved Kedrion Biopharma’s and Kamada’s KEDRAB (rabies immune globulin [human]) for passive, transient postexposure prophylaxis of rabies infection.
Novartis’ Kymriah (tisagenlecleucel) has been approved by the U.S. Food and Drug Administration to treat pediatric acute lymphoblastic leukemia.
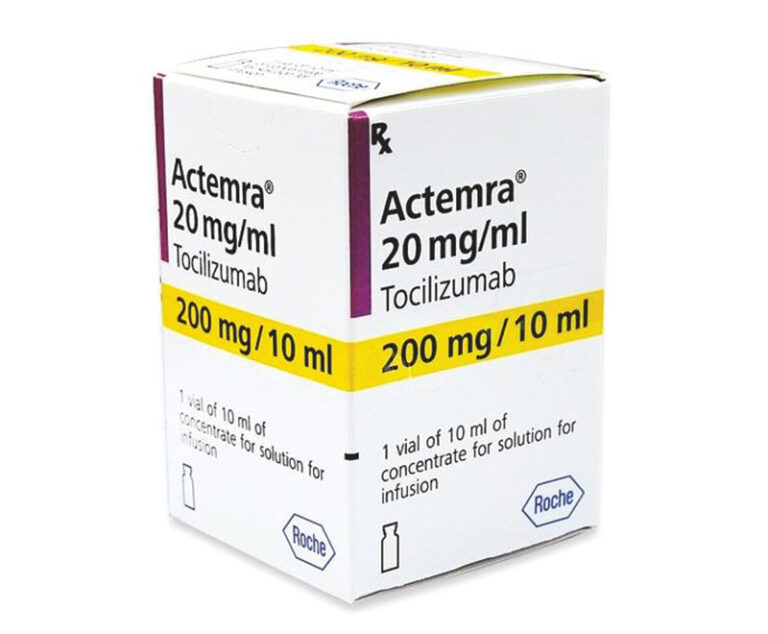
The U.S. Food and Drug Administration has approved Roche’s Actemra (tocilizumab), the first treatment for adult patients with giant cell arteritis.
The U.S. Food and Drug Administration has approved new product strengths for Octapharma’s NUWIQ.