Industry News
Research, Science & Manufacturer Updates
Medicines Articles
The U.S. Food and Drug Administration has approved Novo Nordisk’s Rebinyn (coagulation factorIX [recombinant], glycopegylated) to treat hemophilia B in adults and children.
The U.S. Food and Drug Administration has approved Renflexis (infliximababda, Samsung Bioepis), the second biosimilar to Remicade (infliximab, Janssen Biotech).
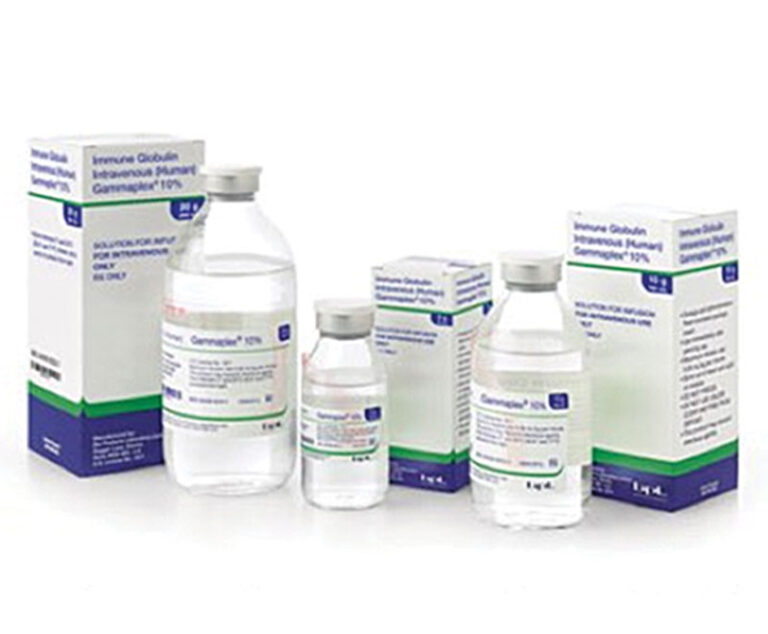
The U.S. Food and Drug Administration has approved Bio Products Laboratory’s Gammaplex 10% (immune globulin intravenous [human] 10% liquid) for the treatment of primary immunodeficiency (PI) and chronic immune thrombocytopenic purpura in adults.
Sandoz announced the U.S. market introduction of rosuvastatin calcium tablets, a generic version of Crestor by AstraZenica Pharmaceuticals LP.
The U.S. Food and Drug Administration approved bezlotoxumab (Zinplava, Merck) to prevent the recurrence of Clostridium difficile (C. diff) infection (CDI) in patients aged 18years and older.
The U.S. Food and Drug Administration has approved Amgen’s Amjevita (adalimumab-atto) as a biosimilar to Humira (adalimumab) for the treatment of several autoimmune conditions.
The U.S. Food and Drug Administration has approved Gilead Sciences’ Vemlidy (tenofovir alafenamide,TAF) 25 mg once-daily treatment for adults with chronic hepatitis B virus(HBV) infection with compensated liver disease.
Results from the Phase III PROLONG9FP ongoing extension clinical development program evaluating the long-term efficacy and safety of IDELVION (coagulation factor IX [recombinant], albumin fusion protein) showed that extended prophylaxis treatment regimens effectively prevented bleeds while also reducing overall IDELVION consumption.
The U.S. Food and Drug Administration granted orphan drug designation to dusquetide (SGX942, Soligenix) for the treatment of macrophage activation syndrome.
The U.S. Food and Drug Administration approved cabozantinib (Cabometyx, Exelixis) to treat advanced renal cell carcinoma (RCC) in patients who have received prior anti-angiogenic therapy.
True North Therapeutics’ TNT009 for the treatment of autoimmune hemolytic anemia, including cold agglutinin disease (CAD), has been granted orphan drug status by the U.S. Food and Drug Administration.
Empliciti (elotuzumab) in combination with Revlimid (lenalidomide) and dexamethasone (a type of corticosteroid) has been approved by the U.S. Food and Drug Administration to treat individuals with multiple myeloma who have received one to three prior medications.