Industry News
Research, Science & Manufacturer Updates
A Phase I clinical trial has shown that NGP 555, a drug developed to treat and prevent Alzheimer’s disease, was safe and well-tolerated with dose-dependent plasma exposure.
Study results from the division of oncology at University Hospital Basel in Switzerland show that patients treated with PD-1/PD-L1 checkpoint inhibitors may be at an increased risk for adverse events after receiving the seasonal influenza vaccination.
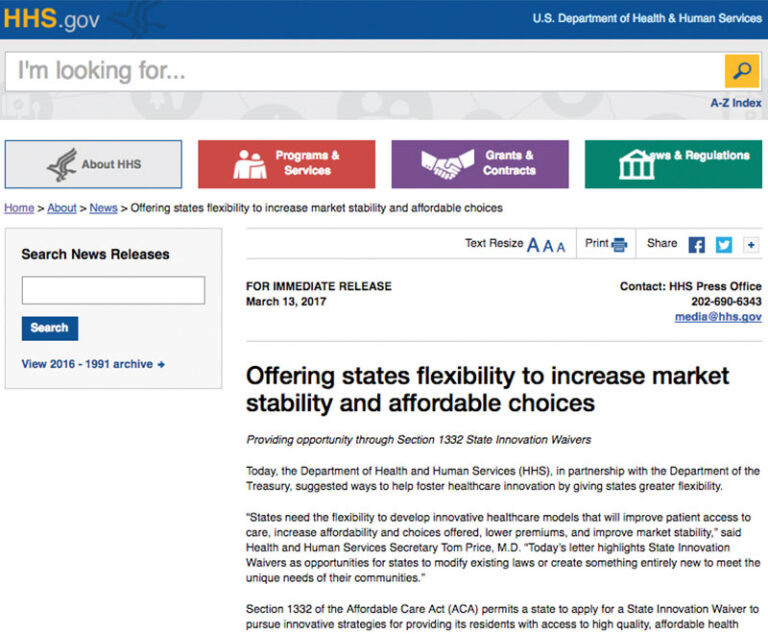
The U.S. Department of Health and Human Services has launched anew page on its website that highlights regulatory and administrative actions it is taking to support a patient-centered healthcare system.
A new study shows newborns of pregnant women who have gotten their pertussis booster vaccine are far less likely to get
the disease than other babies.
Connected Care is a new educational initiative to raise awareness of the benefits of chronic care management (CCM)services for Medicare beneficiaries with multiple chronic conditions.
A new vaccine to protect against rotavirus has been developed by Serum Institute of India.
Administration of high-dose intravenous immune globulin (IVIG) significantly improved muscle and joint pain, muscle weakness and markers of systemic inflammation, according to a retrospective evaluation of 46 systemic sclerosis patients at 19 French centers.
The U.S. Food and Drug Administration has approved Renflexis (infliximababda, Samsung Bioepis), the second biosimilar to Remicade (infliximab, Janssen Biotech).
Under a new rule, the Health Resources and Services Administration of the Department of Health and Human Services can issue fines of up to $5,000 for each incident to drug manufacturers that knowingly and intentionally overcharge 340B hospitals for drugs purchased under the program.
Researchers at Brigham and Women’s Hospital have discovered a subset of T cells that collaborate with other immune cells to drive inflammation in peripheral tissues.
Researchers at the Moffitt Cancer Center are developing a new vaccine that will help early-stage breast cancer patients who have HER2 positive disease.
The Centers for Medicare and Medicaid Services (CMS) issued an interim rule to delay the implementation of its bundled payment program for cardiac care, as well as for expansion of its Comprehensive Care for Joint Replacement (CJR) program.