Industry News
Research, Science & Manufacturer Updates
Immune Globulin Articles
Mylan’s Ogivri (trastuzumab-dkst) has been approved by the U.S. Food and Drug Administration as a biosimilar to Genentech’s Herceptin (trastuzumab).
A Phase III, placebo-controlled study demonstrated both low-dose and high-dose therapy with a licensed, self-administered subcutaneous immune globulin product was efficacious and well-tolerated as maintenance treatment for patients with chronic inflammatory demyelinating polyneuropathy.
The U.S. Food and Drug Administration (FDA) has approved Privigen (immune globulin intravenous [human] 10% liquid) to treat adults with chronic inflammatory demyelinating polyneuropathy (CIDP) to improve neuromuscular disability.
Canadian investigators at the University of Alberta evaluated the use of self-administered subcutaneous immune globulin in a prospective, open-label, Phase III crossover trial in adult patients with myasthenia gravis experiencing mild to moderate worsening of symptoms.
Australian investigators tested the ability of intravenous immune globulin to protect against potential pandemic influenza virus in outbred ferrets, which are naturally susceptible to human influenza viruses and considered a relevant small animal model of human influenza infection.
Administration of high-dose intravenous immune globulin (IVIG) significantly improved muscle and joint pain, muscle weakness and markers of systemic inflammation, according to a retrospective evaluation of 46 systemic sclerosis patients at 19 French centers.
A new study shows that individuals treated with intravenous immune globulin (IVIG) for Guillain-Barré syndrome are at increased risk of developing hypoalbuminemia (reduced albumin levels).
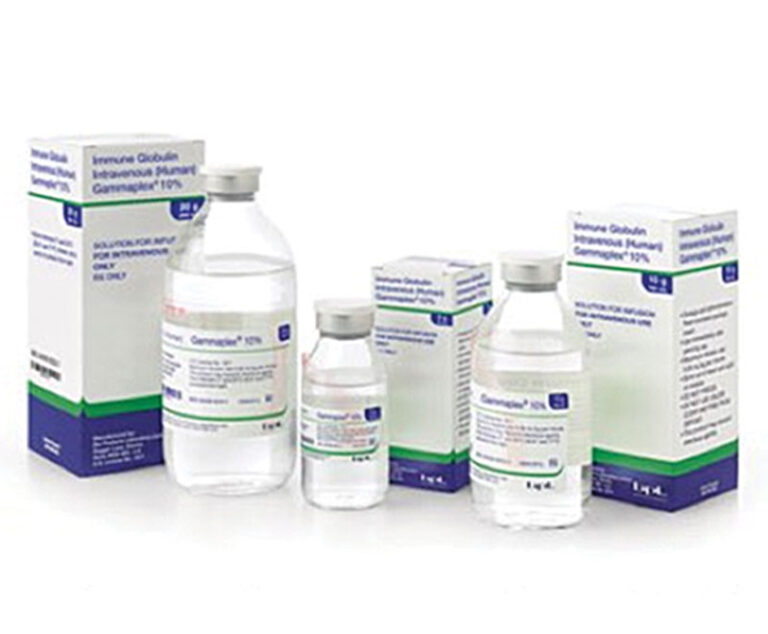
The U.S. Food and Drug Administration has approved Bio Products Laboratory’s Gammaplex 10% (immune globulin intravenous [human] 10% liquid) for the treatment of primary immunodeficiency (PI) and chronic immune thrombocytopenic purpura in adults.
A recent study discovered a biomarker in chronic inflammatory demyelinating polyneuropathy patients that explains why they don’t respond to intravenous immune globulin therapy.
The U.S. Food and Drug Administration ha approved Octapharma’s NUWIQ, antihemophilic factor (recombinant), an intravenous therapy for adults and children living with hemophilia A.
The U.S. Food and DrugAdministration (FDA) approved BioProducts Laboratory’s Gammaplex(immune globulin intravenous [human]5% liquid) for pediatric patients 2 years of age and older who have primary immunodeficiency disease (PI).
Pfizer (a licensee of Gliknik Inc.) has been granted orphan drug designation by the U.S. Food and Drug Administration (FDA) for its recombinant intravenous immune globulin (IVIG)-mimetic drug
GL-2045 to treat chronic inflammatory demyelinating polyneuropathy (CIDP).