Industry News
Research, Science & Manufacturer Updates
Therapeutics Articles
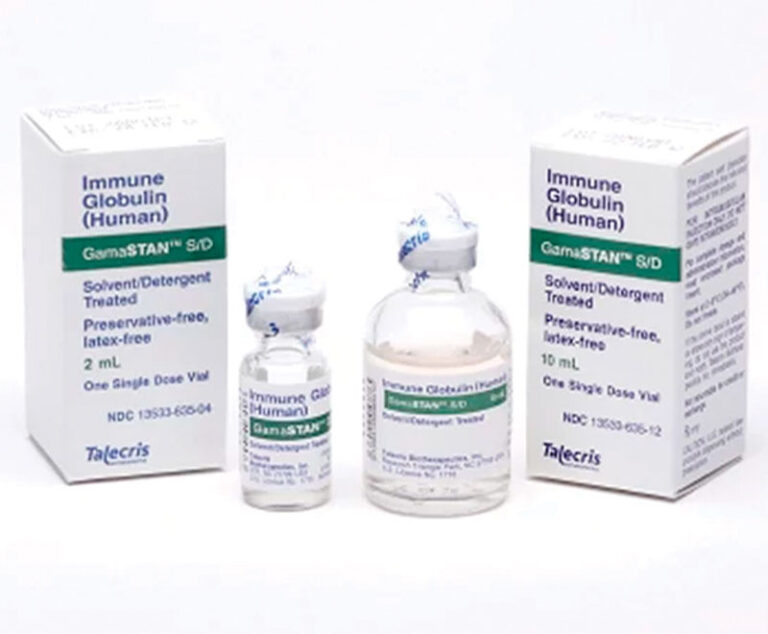
FDA has approved Grifols’ GamaSTAN to treat people exposed to measles and the hepatitis A viruses.
Subcutaneous administration of lanadelumab, an investigational monoclonal antibody intended for the prevention of attacks in patients with type I or II hereditary angioedema (HAE), significantly reduced the mean number of attacks compared to placebo treatment.
The U.S. Food and Drug Administration (FDA) has approved Bayer’s Jivi (BAY94-9027) as a preventive treatment for bleeding in hemophilia A.
Investigators from the Belgian biotechnology company argenx and U.S. collaborators conducted a PhaseI clinical study to assess a novel modified antibody Fc fragment (efgartigimod) that reduces the circulating IgG level by blocking neonatal Fc receptor-mediated IgG recycling.
The U.S. Food and Drug Administration (FDA) has approved Shire’s Cinryze (C1 esterase inhibitor [human]) to prevent angioedema attacks in children 6 years and older with hereditary angioedema.
The U.S. Food and Drug Administration (FDA) has approved Incyte Corp.’s Olumiant (baricitinib) 2 mg once-daily oral medication to treat adults with moderately to severe active rheumatoid arthritis.
The U.S. Food and Drug Administration has approved the first prescription drug made from marijuana.
The U.S. Food and Drug Administration has approved Genentech’s Rituxan (rituximab) to treat moderate-tosevere phemphigus vulgaris.
The U.S. Food and Drug Administration (FDA) has approved a new, single-dose medication to treat people 12 years and older who have had the flu for no more than 48 hours.
The U.S. Food and Drug Administration has approved Erleada (apalutamide) to treat men with prostate cancer that has not yet spread but has a quickly rising PSA level while on treatment with hormone therapy, which causes concern for cancer growth and spread.
The Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) have updated guidelines for diagnosis and management of Clostridium difficile (C. diff).
A longitudinal study of Canadian boys with severe hemophilia showed that tailored frequency-escalated prophylaxis results in minimal long-term arthropathy and very good health outcomes, while reducing the quantity of costly clotting factor as compared with standard prophylaxis protocols.