Industry News
Research, Science & Manufacturer Updates
Therapeutics Articles
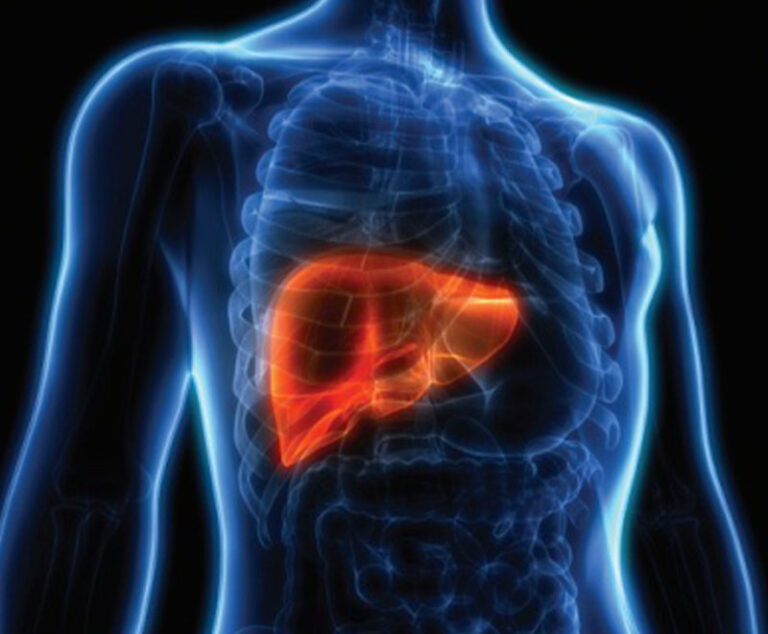
Results from a clinical trial have led investigators to conclude adding longterm administration of human albumin to conventional treatment inpatients with decompensated cirrhosis appears to prolong survival.
The Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) have updated guidelines for diagnosis and management of Clostridium difficile (C. diff).
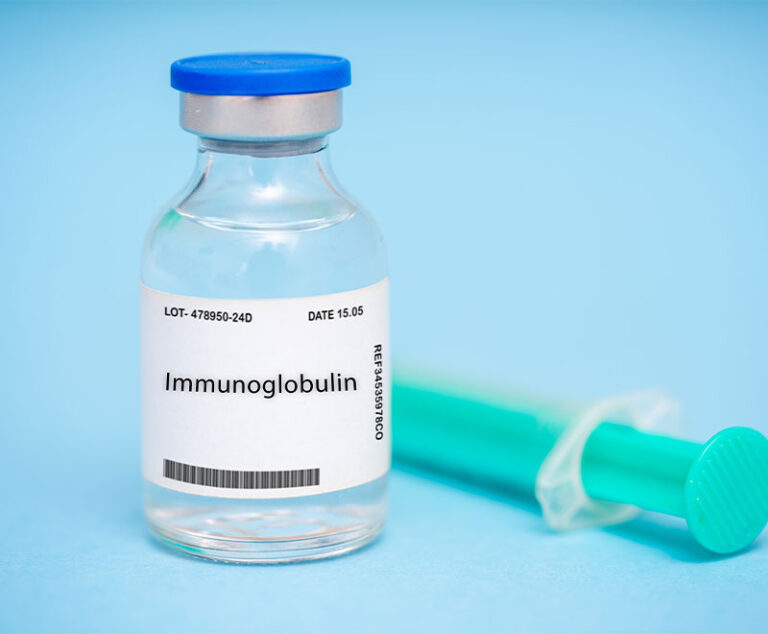
Grifols’ higher-potency rabies immune globulin (RIG), HyperRAB S/D, was made available to healthcare providers
Discontinuation of the product is due to the preference among healthcare professionals and patients for newer, more advanced immune globulin options.
The U.S. Food and Drug Administration has approved Shire’s VONVENDI, a recombinant von Willebrand factor treatment for perioperative management of bleeding in adults 18 years and older with von Willebrand disease.
Researchers have found antibiotics can be counterproductive and weaken the immune system’s ability to fight off bacteria.
A multidisciplinary team of investigators based in New Delhi randomized 120 patients with liver cirrhosis and overt hepatic encephalopathy (HE) to receive oral lactulose therapy or oral lactulose plus 1.5 g/kg/day of human albumin. The primary study endpoint was complete reversal of HE; secondary endpoints included mortality and length of hospital stay.
Australian investigators tested the ability of intravenous immune globulin to protect against potential pandemic influenza virus in outbred ferrets, which are naturally susceptible to human influenza viruses and considered a relevant small animal model of human influenza infection.
Grifols has made a major equity investment in Alkahest to work together to develop novel plasma-based products for the treatment of cognitive decline in aging and disorders of the central nervous system.
A recent study shows that a vaccine typically used to prevent tuberculosis in countries outside of the U.S. could also prevent multiple sclerosis (MS) in people who are in the beginning stages of the disease.
Researchers at King’s College London have identified a new gene, PIM1, which could be an effective target for innovative treatments and therapies for psoriasis, an autoimmune disease.
A recent study of an experimental therapy that alters cancer patients’ own immune cells to recognize an often deadly form of leukemia has shrunk tumors and sent the cancer into remission in adults.