Industry News
Research, Science & Manufacturer Updates
Therapeutics Articles
Researchers have discovered a rare type of immune cell that may predict how likely some patients with skin cancer will respond to immunotherapy treatment.
Imperial College Healthcare NHS Trust has enrolled the first United Kingdom (UK) patients who have received an experimental messenger RNA (mRNA) therapy — a type of immunotherapy treatment called mRNA-4359 — in its Phase I/II clinical trial.
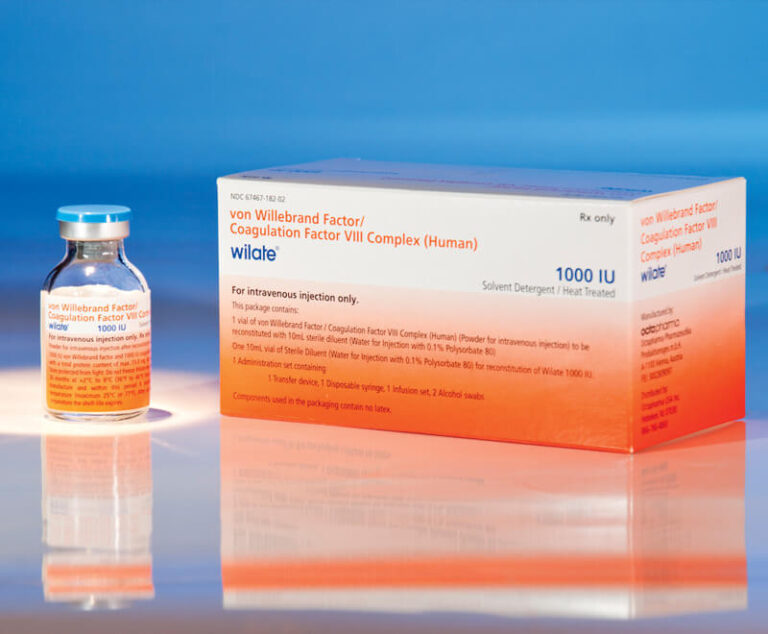
Octapharma USA's wilate, von Willebrand factor (VWF)/coagulation factor VIII complex (human) lyophilized power for solution for intravenous injection, has been given expanded approval by the U.S. Food and Drug Administration (FDA) for routine prophylaxis to reduce the frequency of bleeding episodes in adults and children aged 6 and older with any type of von Willebrand disease (VWD).
In a study, researchers found that fSCIG 10% was more effective in preventing CIDP relapse than placebo, supporting its potential use as maintenance CIDP treatment.
A team of Australian investigators conducted a retrospective cohort study to determine whether there is a significant benefit in more severely affected cases regularly infused with albumin.
An international early-phase clinical trial has found a "two-for-one" cancer immunotherapy, tebotelimab, is potentially more effective and at least as safe as standard immunotherapies.
Pfizer's PENBRAYA, a vaccine for meningococcal groups A, B, C, W and Y, has been approved by the U.S. Food and Drug Administration (FDA).
The U.S. Food and Drug Administration (FDA) has approved Celltrion's Zymfentra, a subcutaneous injection formulation of its infliximab Remsina, for maintenance therapy in adults with moderately to severely active ulcerative colitis and Crohn's disease following treatment with an infliximab administered intravenously.
The U.S. Food and Drug Administration (FDA) has approved Wezlana (ustekinumab-auub) as a biosimilar to and interchangeable with Stelara for adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy; active psoriatic arthritis; moderately to severely active Crohn's disease; and moderately to severely active ulcerative colitis.
Investigators at the Icahn School of Medicine at Mount Sinai have designed an RNA-based strategy to activate dendritic cells, which play a key role in immune response, that eradicated tumors and prevented their recurrence in mouse models of melanoma.
The combination of intravenous immune globulin (IVIG) and corticosteroids is a more efficient and rapid treatment for relapsed immune thrombocytopenic purpura (ITP) in adults compared with the use of either therapy alone.
A study has found that early albumin administration in septic shock patients with ARDS was independently associated with a reduction in 28-day mortality.