Industry News
Research, Science & Manufacturer Updates
Washington Report Articles
The U.S. Food and Drug Administration (FDA) issued a final rule to require new health warnings on cigarette packages and in cigarette advertisements, which feature textual statements with photo-realistic color images depicting some of the lesser-known, but serious health risks of cigarette smoking, including impact to fetal growth, cardiac disease, diabetes and more.
The Centers for Medicare and Medicaid Services (CMS) has broadened access to Medicare telehealth services so beneficiaries can receive a wider range of services from their doctors without having to travel to a healthcare facility.
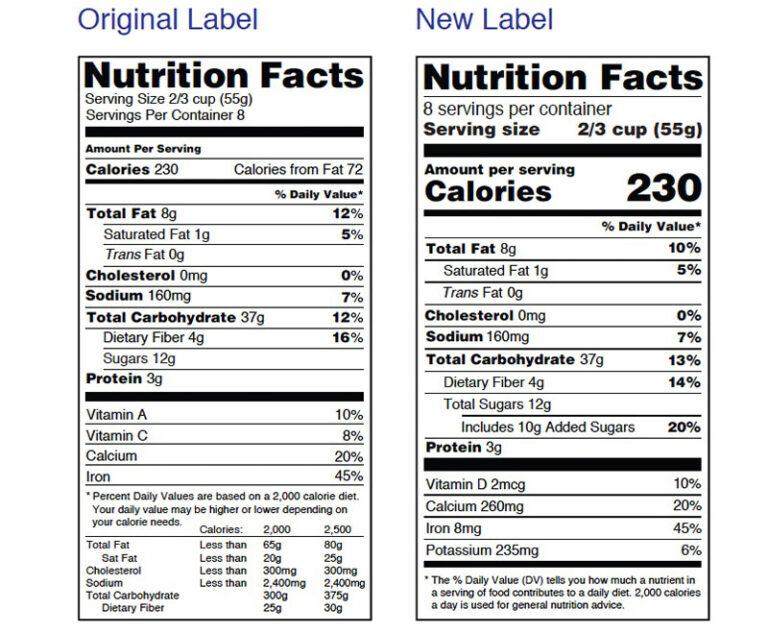
The U.S. Food and Drug Administration (FDA) has launched a campaign to help consumers use the new Nutrition Facts label that appears on packaged foods to maintain healthy dietary practices.
In March, the U.S. Department of Health and Human Services (HHS) finalized two rules that give patients unprecedented safe and secure access to their health data.
The Centers for Medicare and Medicaid Services (CMS) has launched the Healthy Adult Opportunity (HAO) optional demonstration initiative to give states tools to design innovative health coverage programs for adult beneficiaries, while holding states accountable for results and maintaining strong protections for the most at-risk populations.
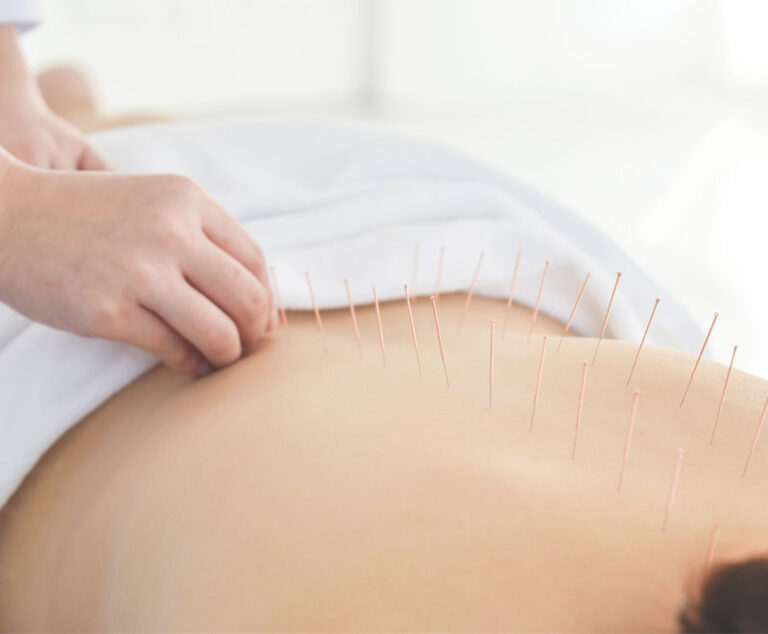
The Centers for Medicare and Medicaid Services (CMS) finalized a decision to cover acupuncture for Medicare patients with chronic low back pain.
New rule requires hospitals to share previously obscured price information, including discounts for cash-paying patients and rates negotiated with insurers.
The U.S. Food and Drug Administration (FDA) is taking critical actions to advance development of novel coronavirus medical countermeasures.
The awards establish and provide up to seven years of support for three Immune Mechanisms of Protection Against Mycobacterium Tuberculosis (IMPAc-TB) Centers to elucidate the immune responses needed to protect against Mtb infection.
A final rule that went into effect Nov. 4 strengthens the Centers for Medicare and Medicaid Services’ ability to stop fraud before it happens.
The Centers for Medicare and Medicaid Services (CMS) has finalized a rule to update and modernize the Programs of All-Inclusive Care for the Elderly (PACE) that reflects updates based upon best practices in caring for frail and elderly individuals.
The U.S. Food and Drug Administration (FDA) released guidelines on the studies companies need to conduct to show their biosimilar is interchangeable with a biologic.