Industry News
Research, Science & Manufacturer Updates
In response to the national measles outbreak, the American Academy of Pediatrics has released updated measles guidelines on its website.
Novo Nordisk has signed an agreement with Janssen Biotech Inc.
CSL Behring’s Biologics LicenseApplication for the marketing authorization of its long-acting fusion protein linking recombinant coagulation factor IX with recombinant albumin (rIX-FP) has been accepted for review by the U.S.
A recent study found no link between the measles vaccine and autism.
GlaxoSmithKline has developed a new shingles vaccine that is 97 percent effective in adults ages 50 years to 70 years.
A growing number of employers and insurers are limiting how much they’ll pay for certain medical services, according to a recent study.
ADMA Biologics has received positive results on the primary and secondary endpoint evaluations from the Phase III trial for its intravenous immune globulin (IVIG) product RI-002 to treat primary immunodeficiency disease(PI).
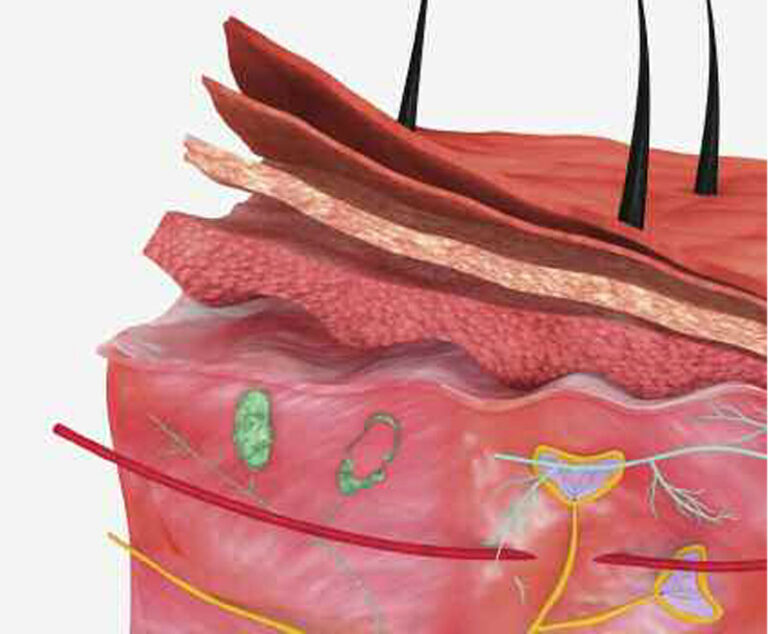
Recent research has found a new approach to stimulate the skin immune response to fight various pathogens with an easy-to-use needle-free vaccine.
The Biosimilars 20/20 Conference, at two-day event that will provide an indepth look into the future of the biosimilars market and address challenges of implementation, is scheduled for June3 and 4 at The Hub in Philadelphia, Pa.
The U.S. Food and Drug Administration(FDA) has approved Octapharma’s manufacturing facility in Vienna, Austria, for the production of Octagam 10% (immune globulin intravenous [human] 10% [100mg/mL] liquid preparation).
The “Albumin in Subarachnoid Hemorrhage” (ALISAH) pilot clinical trial, conducted at the Baylor College of Medicine in Houston, assessed the neuroprotective effects of varying dosages of 25% human albumin. Vasospasm, delayed cerebral ischemia (DCI) and cerebral infarction were evaluated in 20 patients who received seven consecutive daily infusions of 0.625 g/kg (Tier 1), 20 who received 1.25 g/kg (Tier 2), and seven who received 1.875 g/kg (Tier 3).
In an established preclinical bleeding model, reversal of coumarin anticoagulation with Kcentra, a four-factor prothrombin complex concentrate (4F-PCC), was shown to be superior to three-factor prothrombin complex concentrates(3F-PCCs), according to findings reported by CSL Behring investigators.