Industry News
Research, Science & Manufacturer Updates
FDA Updates Articles
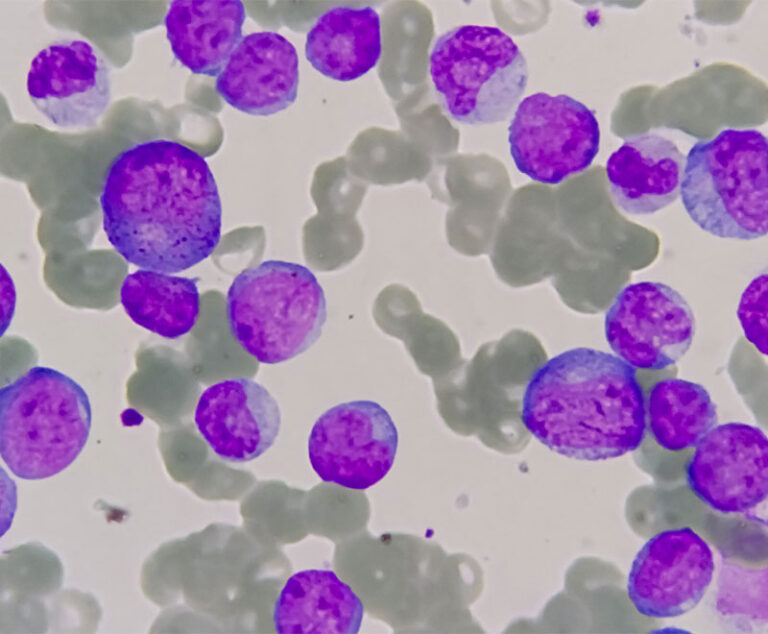
The U.S. Food and Drug Administration has approved Agios Pharmaceuticals’ Idhifa (enasidenib) to treat acute myelogenous leukemia.
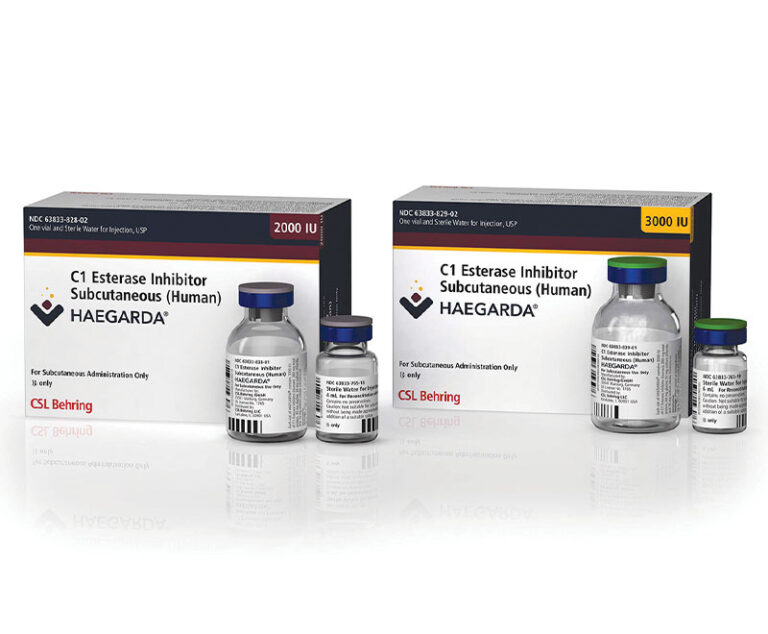
The U.S. Food and Drug Administration has approved CSL Behring’s Haegarda (C1 esterase inhibitor subcutaneous [human]), the first and only subcutaneous therapy indicated for routine prophylaxis to prevent hereditary angioedema attacks in adolescent and adult patients.
The U.S. Food and Drug Administration (FDA) has approved Privigen (immune globulin intravenous [human] 10% liquid) to treat adults with chronic inflammatory demyelinating polyneuropathy (CIDP) to improve neuromuscular disability.
The U.S. Food and Drug Administration has approved AbbieVie Inc.’s Mavyret (glecaprevir and pibrentasvir) to treat adults with certain types of hepatitis C.
The U.S. Food and Drug Administration has approved Kedrion Biopharma’s and Kamada’s KEDRAB (rabies immune globulin [human]) for passive, transient postexposure prophylaxis of rabies infection.
Novartis’ Kymriah (tisagenlecleucel) has been approved by the U.S. Food and Drug Administration to treat pediatric acute lymphoblastic leukemia.
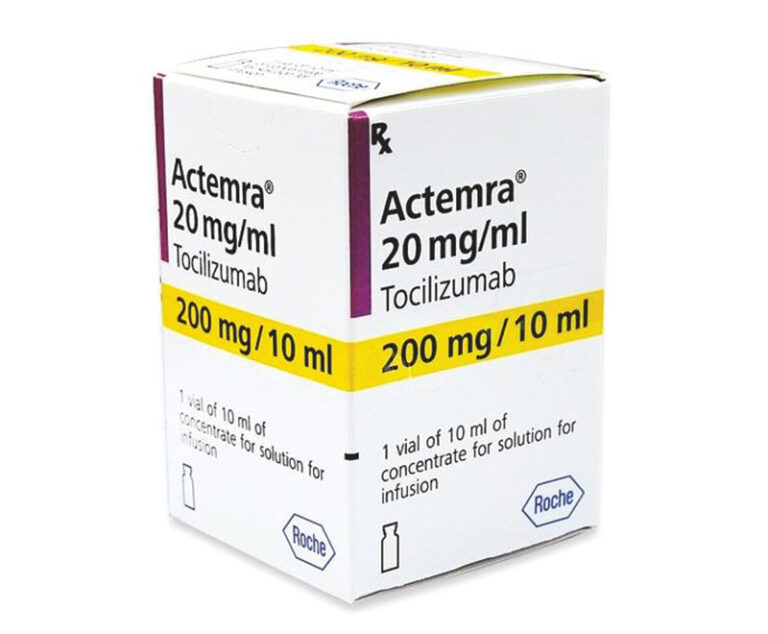
The U.S. Food and Drug Administration has approved Roche’s Actemra (tocilizumab), the first treatment for adult patients with giant cell arteritis.
The U.S. Food and Drug Administration has approved new product strengths for Octapharma’s NUWIQ.
The U.S. Food and Drug Administration has approved Novo Nordisk’s Rebinyn (coagulation factorIX [recombinant], glycopegylated) to treat hemophilia B in adults and children.
The U.S. Food and Drug Administration has approved Renflexis (infliximababda, Samsung Bioepis), the second biosimilar to Remicade (infliximab, Janssen Biotech).
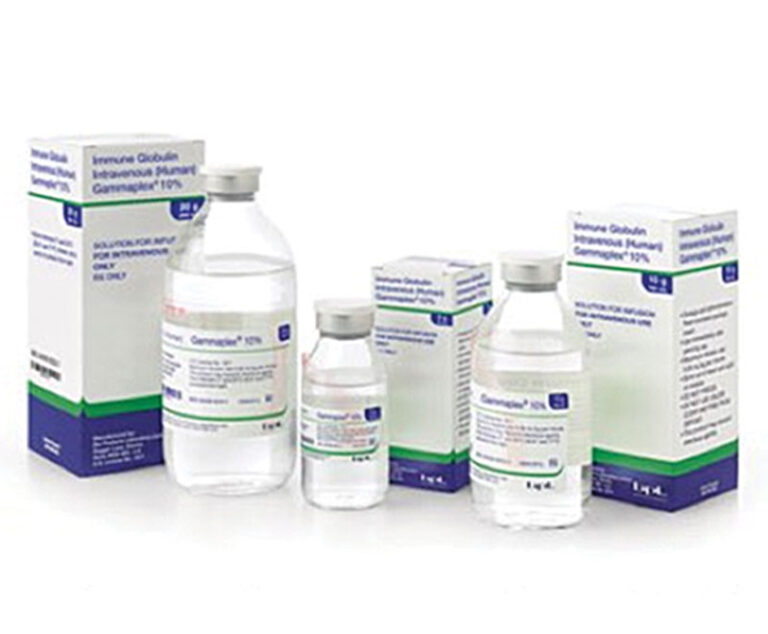
The U.S. Food and Drug Administration has approved Bio Products Laboratory’s Gammaplex 10% (immune globulin intravenous [human] 10% liquid) for the treatment of primary immunodeficiency (PI) and chronic immune thrombocytopenic purpura in adults.
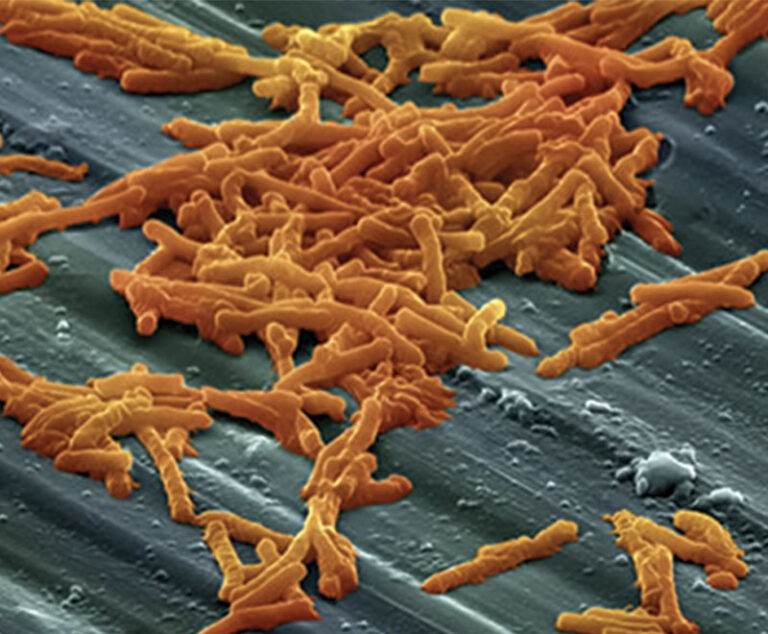
The U.S. Food and Drug Administration approved bezlotoxumab (Zinplava, Merck) to prevent the recurrence of Clostridium difficile (C. diff) infection (CDI) in patients aged 18years and older.