Industry News
Research, Science & Manufacturer Updates
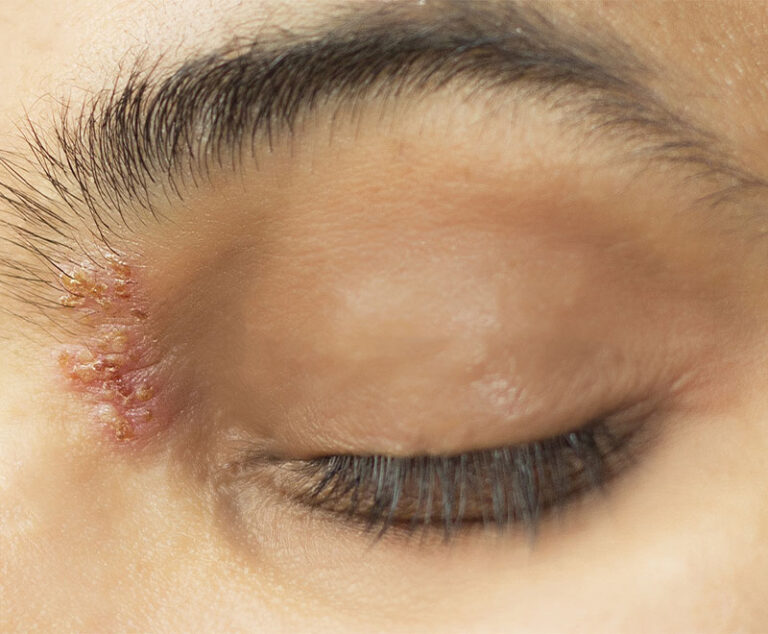
GlaxoSmithKline (GSK) has receivedU.S. Food and Drug Administration approval for its Shingrix vaccine to prevent shingles (herpes zoster) in patients 50 years and older.
The U.S. Food and Drug Administration has approved Kedrion Biopharma’s and Kamada’s KEDRAB (rabies immune globulin [human]) for passive, transient postexposure prophylaxis of rabies infection.
Novartis’ Kymriah (tisagenlecleucel) has been approved by the U.S. Food and Drug Administration to treat pediatric acute lymphoblastic leukemia.
The National Institutes of Health’s National Institute of Allergy and Infectious Diseases division has awarded nearly $9 million to researchers from Children’s Center for Cancer and Blood Diseases at Children’s Hospital Los Angeles and Boston Children’s Hospital to study the lowest dose of chemotherapy needed for babies with severe combined immunodeficiency(SCID) undergoing bone marrow transplant, the standard treatment for SCID.
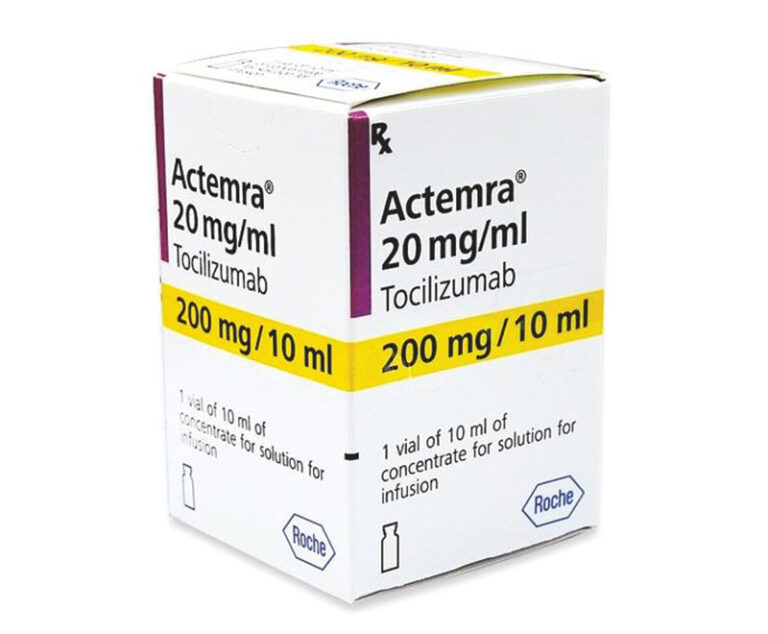
The U.S. Food and Drug Administration has approved Roche’s Actemra (tocilizumab), the first treatment for adult patients with giant cell arteritis.
The U.S. Supreme Court ruled that faith-based hospitals’ pension plans qualify for the “church plan” exemption from the Employee Retirement Income Security Act (ERISA).
The U.S. Food and Drug Administration has approved new product strengths for Octapharma’s NUWIQ.
A clinical trial comparing the protective efficacy in older adults of a quadrivalent recombinant influenza vaccine with a standard-dose, egg-grown quadrivalent inactivated influenza vaccine during the A/H3N2-predominant 2014-2015 influenza season showed RIV4 provided better protection against confirmed influenza-like illness among older adults.
The Agency for Healthcare Research and Quality has released a preliminary update to the National Scorecard on Rates ofHospital-Acquired Conditions that show a 21 percent reduction in hospital-acquired conditions from 2010 to 2015.
The Centers for Medicare and Medicaid would exempt physician practices with less than $90,000 in Medicare revenue or fewer than 200 unique Medicare patients per year from complying with the Medicare Access and CHIP Reauthorization Act (MACRA) of 2015.
Sanofi Pasteur has acquired Protein Sciences, a privately held vaccines biotechnology company based inMeriden, Conn.
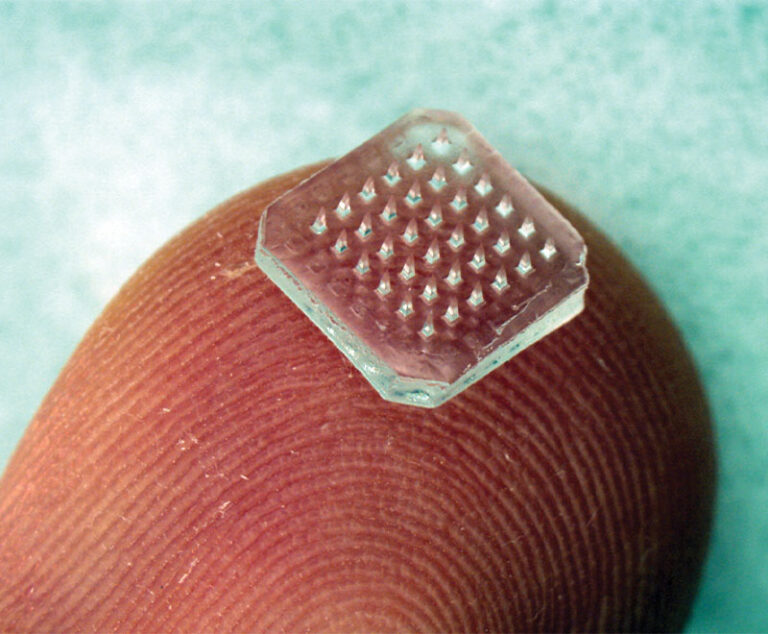
A new study shows that microneedle patches provide an alternative to conventional needle-and-syringe immunization, potentially offering improved immunogenicity, simplicity, cost-effectiveness, acceptability and safety.